INTRODUCTION
Breast reconstruction is part of a multidisciplinary approach to treat breast
cancer. It can be performed using flaps (autologous tissue) and breast implants
alone or in combination.
Immediate breast reconstruction (IBR) follows mastectomy. Collaboration between
the mastologist and plastic surgeon is necessary. Advantages include a single
hospital stay, a single operation under anesthesia, reduced hospital costs, ease
of exposure of the surgical field by mastectomy for reconstruction,
aesthetically restored balance, and psychological reassurance for the
patient1,2.
In 1984, Becker advocated the use of a permanent expander, combining the benefits
of silicone gel, saline implant, and expander implant into a single product,
which could allow breast reconstruction in a single step.
The definitive Becker expander has a double lumen and a textured external surface
and contains silicone gel as 35% of the expander volume in the anterior layer,
with a posterior chamber for an additional 65% that can be filled with saline
solution using an external valve3. IBR
using the breast expander is simpler and faster, with shorter convalescence time
compared to other reconstruction techniques4,5.
Despite being a simple method for breast reconstruction, the use of the Becker
expander has unique features and like any technique is associated with some
complications.
Late complications may be intense and deforming in a minority of patients and may
be a cause of pain, especially after radiotherapy6. Other complications include seroma formation, infections, and
tissue necrosis resulting from the surgical trauma of mastectomy7,8. Inconveniences include the acquisition and cost of expanders,
quality control, risk of elastomer rupture, and need for outpatient follow-up
for expansion sessions9.
Many implants are available, but in patients with very large breasts, no implants
have sufficient volume for adequate reconstruction.
The original proposal of this study is the use of the Becker expander combined
with a second breast implant. The Becker expander is the basis for the second
implant, thus determining the final volume of the reconstructed breast.
OBJECTIVE
The objective of this work is to present a new proposal for breast reconstruction
using the Becker 35 expander in large-volume breast reconstruction.
METHODS
This retrospective study examined the medical records of the senior author’s
patients. The following inclusion criteria were established: patients who had
undergone breast reconstruction using the Becker Expander 35, in which posterior
breast symmetrization had been performed, but the volume of the new implant was
insufficient for aesthetic adequacy even with the reduction in the size of the
opposite breast. The study period was between January 2014 and October 2016.
The consultations, diagnostic investigation, surgical treatment, adjuvant
oncological treatment, and clinical oncological follow-up were performed at the
Hospital AC Camargo - Cancer Center of Fundação Antônio Prudente - São Paulo,
SP.
The patients were followed-up by a multidisciplinary team from the Hospital do
Câncer that included professionals from the following specialties:
Plastic Surgeons - Performed evaluation of the patient’s
psychological motivation and general and specific clinical and physical
conditions relevant to reconstruction, such as contralateral breast
conditions, scars, shape, and volume of the breasts. The donor areas
were determined with the participation of the patient.
Mastologists - Were involved in staging, on the basis of
clinical parameters, imaging, and anatomic-pathologic assessment
according to the TNM classification (Union for International Cancer
Control). The mastologist performed mastectomy and was involved in
ongoing oncological management.
Radiotherapists - Assisted in follow-up, methodology, and
management of complications.
Clinical Oncologists - Were involved in the evaluation of
clinical conditions, choice of drugs/doses, and management of
complications of chemotherapy.
Anesthetists - Performed preoperative evaluation and
determined the anesthetic technique.
Pathologists - Performed all tissue examinations including
immunohistochemistry.
Psychiatrists - Provided support for mastectomy
patients.
Physiotherapists - Enabled upper limb functional recovery
after mastectomy.
A Dressing group - Provided nursing with specific techniques
for use in breast reconstruction.
The expander is placed in submuscular position, following dissection of the
pectoralis major, with additional coverage using the serratus anterior and
anterior sheath of the rectus abdominis when needed.
Dissection was performed with reference to the original submammary sulcus and the
anatomic limits of the breast. This ensures adequate coverage for the expander.
The volume of the expander is determined by reference to the contralateral
breast and the weight of the mastectomy product.
When implants overlapped, the Becker expander was completely emptied prior to
removal of the valve and adjustments were performed when necessary.
Vacuum drainage was routinely used.
When the proposed technique was used in the second surgical step, the procedure
was as follows. The expander was completely emptied (Figure 1), maintaining only the silicone, i.e., 35% of the
original volume of the expander (Figure 2).
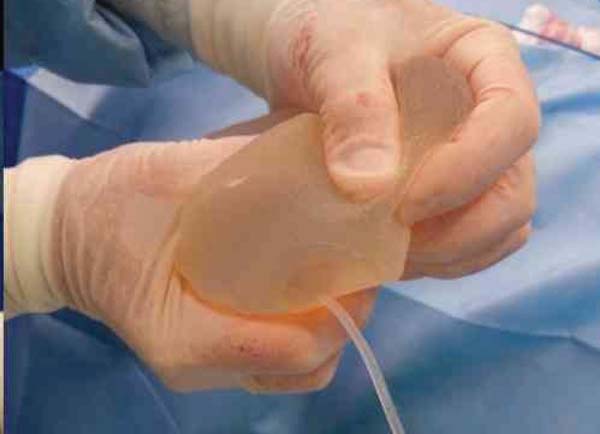
Figure 1 - Emptying the Becker expander Implant. The catheter attached to
the valve is removed using an internal sealing system.
Figure 1 - Emptying the Becker expander Implant. The catheter attached to
the valve is removed using an internal sealing system.
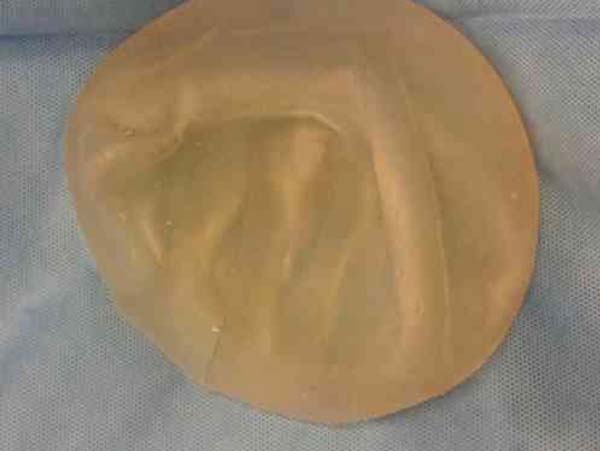
Figure 2 - Empty Becker Expander Implant. Note the presence of gel at the
top of the implant and the empty bottom acting as the base.
Figure 2 - Empty Becker Expander Implant. Note the presence of gel at the
top of the implant and the empty bottom acting as the base.
When the expander was drained, the valve and connector were also removed. The
Becker expander was used as a basis to support a conventional breast implant
with a round (Figure 3) or anatomical
profile (Figure 4).
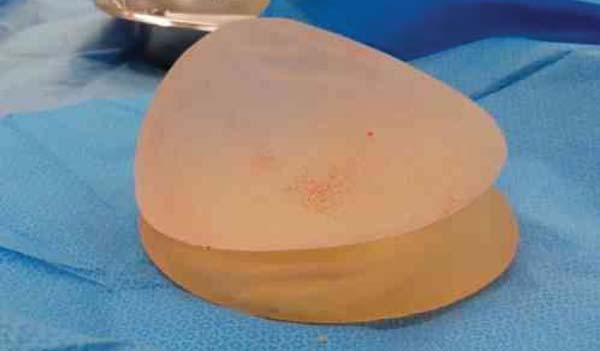
Figura 3 - Round conventional implant on a Becker expander.
Figura 3 - Round conventional implant on a Becker expander.
Figura 4 - Anatomic implant on a Becker expander base.
Figura 4 - Anatomic implant on a Becker expander base.
The study was approved by the institutional ethics committee.
RESULTS
Of 168 patients who underwent IBR, 25 had a bilateral procedure, for a total of
193 breast reconstructions. The mean age was 47 years; 49 patients were smokers,
57 had systemic arterial hypertension, 22 had diabetes, 9 had heart disease, and
5 had other diseases.
Complications in this group included seroma in 7 patients, hematoma in the
mastectomy flap in 2, partial necrosis of the mastectomy flap in 3, and
necrosis/infection with expander exposure in 2. One or more complications
occurred in 9.5% of all cases.
One or more clinical aggravating factors were present in 33% of patients
undergoing mastectomy and immediate reconstruction.
TNM stages ranged from Tis / T1n0m0 to T4n2m0. The mean surgical time for breast
reconstruction was 1 hour.
Regarding adjuvant treatment, 119 (62%) patients had postoperative radiotherapy
and chemotherapy and 83 received chemotherapy alone.
Only plastic surgery was performed in the second surgical phase of breast
reconstruction. In this second phase, reconstruction of the papillary plate and
mammaplasty for contralateral breast symmetrization were performed, when
necessary, in unilateral cases, with or without the use of breast implants.
A total of 133 (69%) patients underwent this second surgical stage to complement
breast reconstruction, with the Becker expander being replaced by a definitive
implant. Complications in this group included seroma in 1 patient, hematoma in
the mastectomy flap in 2, contralateral mammaplasty hematoma in 2, marginal
necrosis in the mastectomy flap in 3, and necrosis/infection with breast implant
exposure in 2 (more than one complication may have occurred in the same
patient). One or more complications associated with the second surgical phase
occurred in 4.5% of cases.
The technique proposed in this study was adopted in 5 patients (3.75%): the empty
Becker expander was used with a second breast implant. There were no
complications in this group. The aim of this technique was to increase the
volume, base, and projection of the reconstructed breast, in which use of a
mammary implant alone would not be satisfactory.
DISCUSSION
Appropriate selection of patients, planning, and good technical execution are
essential for a good result in breast reconstruction without complications10,11. However, patient characteristics such as large breast
volume, a wide breast base, and obesity are challenges often encountered by the
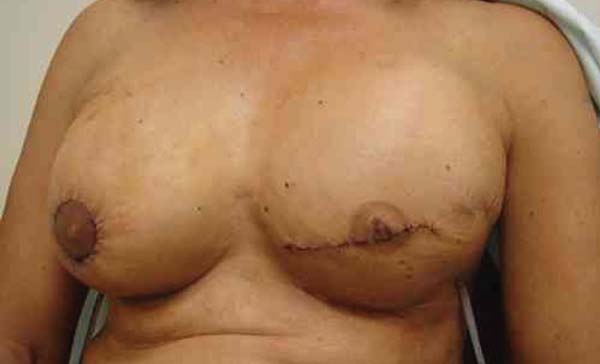
plastic surgeon (Figuras 5, 6 e 7).
Figure 5 - Patient 1 - Right breast reconstructed with the described
technique - Empty Becker Expander implant with the superimposed
round high-profile implant. Note the similar widths of the breast
bases.
Figure 5 - Patient 1 - Right breast reconstructed with the described
technique - Empty Becker Expander implant with the superimposed
round high-profile implant. Note the similar widths of the breast
bases.
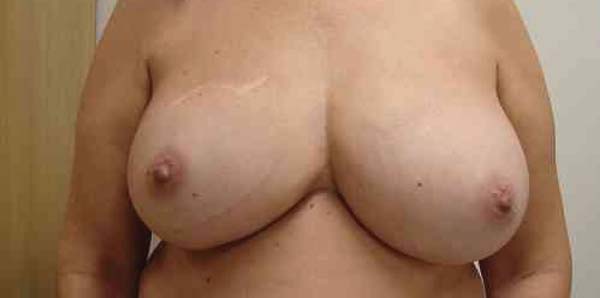
Figure 6 - Patient 2 - Left breast reconstructed with the described
technique - Empty Becker expander with the superimposed round
high-profile implant.
Figure 6 - Patient 2 - Left breast reconstructed with the described
technique - Empty Becker expander with the superimposed round
high-profile implant.
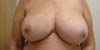
Figure 7 - Patient 3 - Right breast reconstructed with the described
technique - Empty Becker expander with the superimposed anatomical
implant.
Figure 7 - Patient 3 - Right breast reconstructed with the described
technique - Empty Becker expander with the superimposed anatomical
implant.
The Becker expander is a very useful tool for breast reconstruction, but its use
should be limited6. Becker’s initial
proposal for reconstruction in a single operation has not proved feasible in the
long run, since many surgical adjustments are necessary to achieve an optimal
outcome12. The volume of the implant
is a factor that often limits its permanence.
Exchange of the expander with a definitive implant is generally well tolerated by
the patient and is an opportunity to make new adjustments for adequate
symmetrization13. In the present
study, the need for capsulotomy was considered, as well as adequacy of lateral
projection of the implant in the thoracic cavity with limited attachment points,
adjustment in the height of the mammary groove, and vertical repositioning of
the implant, since the expander tends to be in a higher thoracic position.
The largest available Becker expander accommodates 685 cc, with temporary
overexpansion of up to 795 cc. The high-volume, high-profile round implant
(Mentor) accommodates 800 cc. In 5 patients in this study, the volume provided
by an available implant would not have been sufficient to reach the desired
volume or sufficient to obtain adequate symmetry. Even if the volume provided by
a single implant was sufficient, the breast base, breast height, and projection
ratios would not have been not adequate in these 5 patients with very bulky
breasts.
We believe that the technical features of the Becker 35 expander provide larger
horizontal/vertical measurements than breast implants, despite smaller anterior
projection, enabling overlap with a second breast implant to obtain more
adequate symmetry.
In the present study, the replacement rate of the expanders was 69%. Chew et
al.8 reported a 68% exchange and/or
removal rate for expanders in 5 years of follow-up. In Brazil, Cammarota et
al.6 reported a 28.57% replacement
rate for expanders combined with implants. In 15.53% of the cases, the procedure
was sufficient, without need for additional methods.
Complications did not occur with this procedure; moreover, no comparison is
available in the literature for the proposed technique.
Adipose tissue grafting is a current proposal in mammary reconstruction
enhancement and has proven to be very effective, as demonstrated by Bezerra et
al.14 However, in cases requiring
large volumetric increases, several approaches may be needed to achieve this
goal. In Brazil, Blumenschein et al.15
reported the possible use of lipoenxertia in breast reconstruction to achieve an
aesthetic volume increase.
CONCLUSION
The use of a Becker 35 implant with a second breast implant was effective in
achieving larger volume in mammary reconstruction in carefully selected
patients. The technique is reproducible and has a low rate of complications.
More studies and longer postoperative follow-up are necessary to reach more
specific conclusions.
COLLABORATIONS
|
AKD
|
Analysis and/or interpretation of data; final approval of the
manuscript; completion of surgeries and/or experiments; writing the
manuscript or critical review of its contents.
|
|
JAJ
|
Final approval of the manuscript; writing the manuscript or critical
review of its contents.
|
REFERENCES
1. Cheville AL, Tchou J. Barriers to rehabilitation following surgery
for primary breast cancer. J Surg Oncol. 2007;95(5):409-18. PMID: 17457830 DOI:
http://dx.doi.org/10.1002/jso.20782
2. Parker PA, Youssef A, Walker S, Basen-Engquist K, Cohen L, Gritz ER,
et al. Short-term and long-term psychosocial adjustment and quality of life in
women under-going different surgical procedures for breast cancer. Ann Surg
Oncol. 2007;14(11):3078-89. DOI: http://dx.doi.org/10.1245/s10434-007-9413-9
3. Becker H. Breast reconstruction using an inflatable breast implant
with detachable reservoir. Plast Reconstr Surg. 1984;73(4):678-83. PMID: 6709750
DOI: http://dx.doi.org/10.1097/00006534-198404000-00031
4. Becker H. The expandable mammary implant. Plast Reconstr Surg.
1987;79(4):631-7. PMID: 3823256 DOI: http://dx.doi.org/10.1097/00006534-198704000-00023
5. Becker H. The permanent tissue expander. Clin Plast Surg.
1987;14(3):519-27. PMID: 3608362
6. Cammarota MC, Lima RQ, Almeida CM, Esteves BP, Curado DMDC, Ribeiro
Júnior I, et al. Reconstrução de mama com expansor de Becker: uma análise de 116
casos. Rev Bras Cir Plast. 2016;31(1):12-8.
7. Farace F, Faenza M, Bulla A, Rubino C, Campus GV. Is mammary
reconstruction with the anatomical Becker expander a simple procedure?
Complications and hidden problems leading to secondary surgical procedures: a
follow-up study. J Plast Reconstr Aesthet Surg. 2013;66(6):741-6. DOI: http://dx.doi.org/10.1016/j.bjps.2013.02.004
8. Chew BK, Yip C, Malyon AD. Becker expander implants: truly a long
term single stage reconstruction? J Plast Reconstr Aesthet Surg.
2010;63(8):1300-4.
9. Camilleri IG, Malata CM, Stavrianos S, McLean NR. A review of 120
Becker per-manent tissue expanders in reconstruction of the breast. Br J Plast
Surg. 1996;49(6):346-51. DOI: http://dx.doi.org/10.1016/S0007-1226(96)90001-6
10. De Vita R, Zoccali G, Buccheri EM, Costantini M, Botti C, Pozzi M.
Outcome Evaluation after 2023 Nipple-Sparing Mastectomies: Our Experience. Plast
Reconstr Surg. 2017;139(2):335e-47e. DOI: http://dx.doi.org/10.1097/PRS.0000000000003027
11. Munhoz AM, Aldrighi CM, Montag E, Arruda EG, Aldrighi JM, Gemperli
R, et al. Clinical outcomes following nipple-areola-sparing mastectomy with
immediate implant-based breast reconstruction: a 12-year experience with an
analysis of patient and breast-related factors for complications. Breast Cancer
Res Treat. 2013;140(3):545-55. PMID: 23897416 DOI: http://dx.doi.org/10.1007/s10549-013-2634-7
12. Eriksen C, Lindgren EN, Frisell J, Stark B. A prospective randomized
study com-paring two different expander approaches in implant-based breast
reconstruction: one stage versus two stages. Plast Reconstr Surg.
2012;130(2):254e-64e. DOI: http://dx.doi.org/10.1097/PRS.0b013e3182589ba6
13. Farace F, Faenza M, Bulla A, Rubino C, Campus GV. Is mammary
reconstruction with the anatomical Becker expander a simple procedure?
Complications and hidden problems leading to secondary surgical procedures: a
follow-up study. J Plast Reconstr Aesthet Surg. 2013;66(6):741-6. DOI: http://dx.doi.org/10.1016/j.bjps.2013.02.004
14. Bezerra FJF, Moura RMG, Maia Neto JD. Lipoenxertia em reconstrução
mamária. Rev Bras Cir Plást. 2013;28(2):241-6. DOI: http://dx.doi.org/10.1590/S1983-51752013000200012
15. Blumenschein AR, Freitas-Junior R, Tuffanin AT, Blumenschein DI.
Lipoenxertia nas mamas: procedimento consagrado ou experimental? Rev Bras Cir
Plást. 2012;27(4):616-22. DOI: http://dx.doi.org/10.1590/S1983-51752012000400025
1. Hospital A.C. Camargo, Cancer Center, São
Paulo, SP, Brazil.
2. Sociedade Brasileira de Cirurgia Plástica, São
Paulo, SP, Brazil.
Corresponding author: Alexandre Katalinic
Dutra, Rua Prof. Antônio Prudente, 211 - Liberdade - São Paulo, SP,
Brazil. Zip Code 01509-010. E-mail: akdutra@uol.com.br
Article received: February 1, 2017.
Article accepted: September 5, 2018.
Conflicts of interest: none.