INTRODUCTION
Breast cancer is an important public health condition, the most prevalent female
cancer excluding non-melanoma skin cancers, and the second cause of cancer death
in women behind only lung cancer.
According to the American Cancer Society, there were an
estimated 316,120 new cases of breast cancer in the United States in 20171. According to data from the National
Cancer Institute, there were an estimated 57,960 new cases of breast cancer in
Brazil in 20162.
The mortality rate is stable in women aged less than 50 years but has been
decreasing in older women, probably due to greater access to information for a
part of the population, early diagnosis, and improved treatment modalities3.
Mastectomy is an essential part of the treatment for breast cancer. The
reconstruction of the breast helps affected women better preserve their
self-esteem and is a right warranted by law in Brazil since 19994. Breast reconstruction does not interfere
with the sequential steps in cancer treatment and does not compromise the
detection of local recurrence5. Several
breast reconstruction techniques have been described, with individualized
assessments defining the technique best suited for each patient.
The use of the latissimus dorsi myocutaneous flap (LDMF) to cover defects caused
by mastectomy was initially described by Tansini in 1906. However, it was not
until 1978 that this flap was used for the reconstruction of the breast by
Bostwick6.
Since then, the LDMF technique has become a mainstay in breast plastic surgery.
The use of silicone breast implants has helped restore the volume of
reconstructed breasts, since the flap itself does not usually provide sufficient
soft parts to recreate the breast mound. Since the latissimus dorsi muscle is
located in the posterior portion of the trunk, its use for breast reconstruction
would usually require a change of decubitus position during the surgical
procedure, which would imply an increase in surgical time.
The performance of this surgery in a lateral decubitus position has the advantage
of eliminating the change of decubitus position, and as a result, the procedure
is shorter. However, anatomical familiarization is required by the surgeon, not
only with regard to the structures to be dissected, but also to the position in
which the flap is sutured to the receptor site and the suitability for better
customization of the remaining breast skin envelope.
The systematization of this procedure in the lateral decubitus position involves
several aspects, from the positioning of the patient on the surgical table to a
description of the details of dissection and customization of the skin island
and envelope. A predetermined sequence is then configured, without change of the
decubitus position, to reduce the duration of the surgery without affecting the
final result.
OBJECTIVE
The objective of this study was to present a surgical systematization with the
description of a series of cases.
METHOD
This was a retrospective primary study conducted using the medical records and
photographic documentation of patients who underwent breast reconstruction with
the LDMF with a silicone implant in the lateral decubitus position between
October 2015 and April 2017. The patients were operated on by the author in
both, a private clinic and two public services where he is a plastic surgeon
(Hospital Napoleão Laureano-PB and Hospital das Clínicas-PE). The principles of
the Declaration of Helsinki, as revised in 2000, and Resolution 196/96 of the
National Health Council were duly followed.
Inclusion criteria
Patients who underwent mastectomy due to breast cancer underwent immediate or
delayed breast reconstruction using the LDMF with a silicone implant in the
single lateral decubitus position between October 2015 and April 2017 and
were operated on by the author were included in this study.
Exclusion criteria
Patients who were smokers, had a body mass index greater than or equal to 30
kg/m2, and/or missed the minimum 3-month postoperative
follow-up were excluded from this study.
Surgical systematization
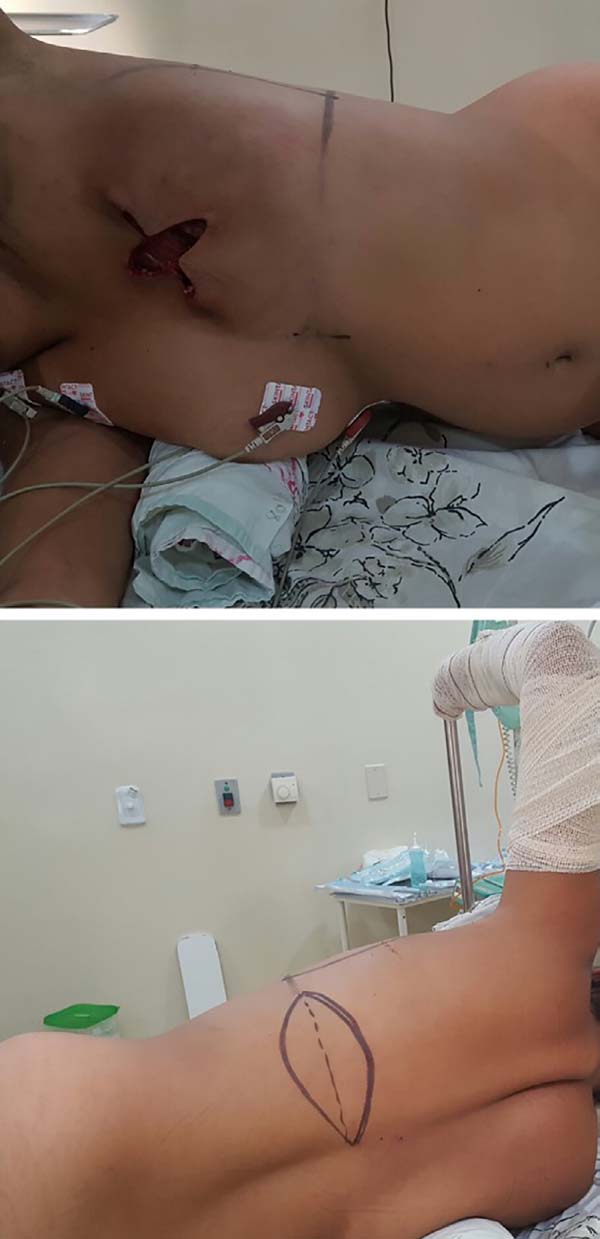
The patient should be positioned in a lateral decubitus position with the
ipsilateral limb abducted at 90 degrees and attached to a fabric-coated arc
through a bandage. Small cushions must be placed below the chest, between
the knees, and to support the head. The hip should be stabilized on the
surgical table with a wide bandage or band suitable for a lateral decubitus
surgery. Thermal blankets and gradual pneumatic-compression leggings are
recommended. The skin island on the dorsum is transversally marked with its
axis following the projection of the corresponding inframammary sulcus,
which ensures that the final scar is hidden in the posterior loop of the
brassiere (Figure 1).
Figura 1 - Positioning of the patient.
Figura 1 - Positioning of the patient.
Further orientations of the skin island can be projected based on the
location of the chest defect. The dimensions usually measure 17 × 7 cm. If
the reconstruction is delayed, surgery is initiated by the removal of the
scar prior to the mastectomy. This is followed by the dissection of the
receptor site at a level along the fascia of the greater pectoral muscle
inferiorly until the projection of the anterior inframammary sulcus,
superiorly up to about 3 cm from the clavicle, medially up to 1.5 cm from
the sternal border, and laterally up to the projection of the midaxillary
line.
In cases of immediate reconstruction, surgery begins with a skin island
incision that preserves some of Scarpa’s fascia superiorly and inferiorly to
the skin island, and then dissecting to the plane closest to the latissimus
dorsi muscle. The dissection should extend medially to the palpation of the
vertebral transverse processes, laterally just beyond the border between the
large dorsal and anterior serratus muscles, and inferiorly until the
identification of the aponeurotic expansion of the latissimus dorsi muscle
near the iliac crest.
The superior dissection is performed later with the aid of the Nelson forceps
and an electric scalpel. The lateral insertion of the latissimus dorsi
muscle is released within the limits already dissected; the muscle is
released to the inferior limit and upon lifting the released muscle, it
becomes easy to incise its origin along the spine. At this time, the muscle
is flat and wide, with its characteristic median thickness.
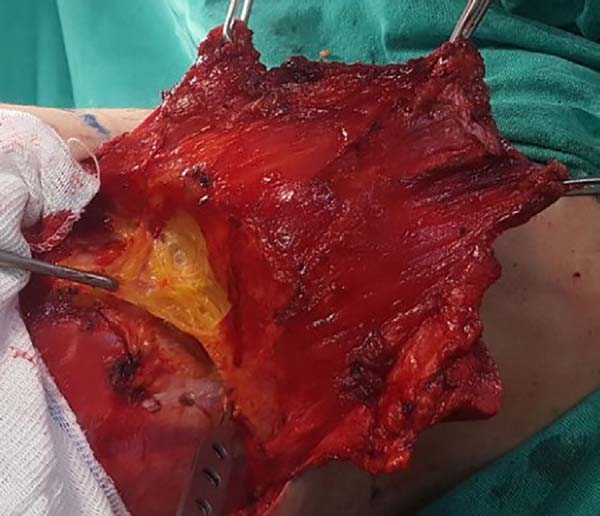
When ascending the dissection, it is important to identify and preserve the
donor site structures that are not necessary for the reconstruction,
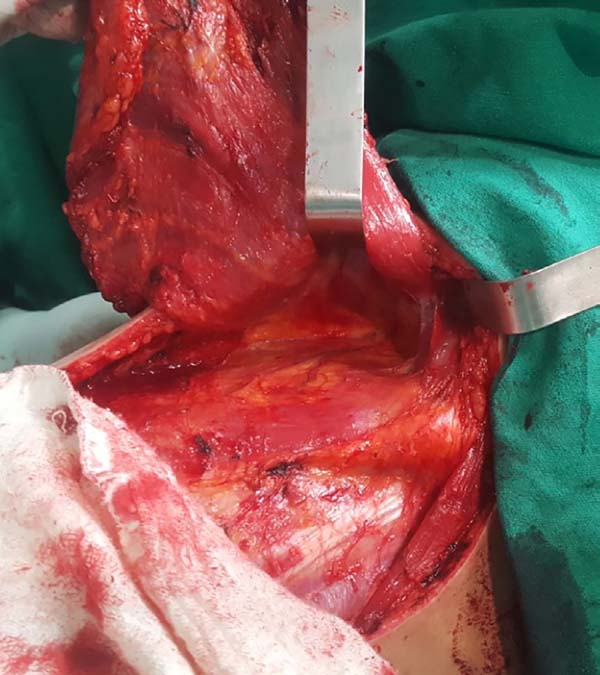
including the fatty fascia behind the latissimus dorsi muscle and the
portion of the trapezius muscle that overlaps medially (Figure 2). Once these structures are preserved, the
dissection of the latissimus dorsi muscle must be complemented up to near
its insertion in the humerus, being its release is optional, with a risk of
torsion of the pedicle in certain cases; the advantage, however, is the
reduced final volume of tissues in the axillary region.
Figura 2 - Fatty fascia behind the latissimus dorsi muscle.
Figura 2 - Fatty fascia behind the latissimus dorsi muscle.
At this time, the muscle should already be fully released in its inferior and
medial portions, requiring only the lateral release, which should be
carefully performed near the axillary region, since the pedicle with the
thoracodorsal vessels is located in this region. The anatomical detail is
that this release should occur over the muscle as the pedicle penetrates
through its deep surface, and the dissection in this deep plane may extend a
little beyond the vascular anatomical finding called the “goose foot”, which
contains branches destined for the anterior serratus muscle and denotes
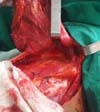
proximity to the thoracodorsal pedicle (Figure 3).
Figure 3 - Elevated flap showing the anatomical finding known as “goose
foot”.
Figure 3 - Elevated flap showing the anatomical finding known as “goose
foot”.
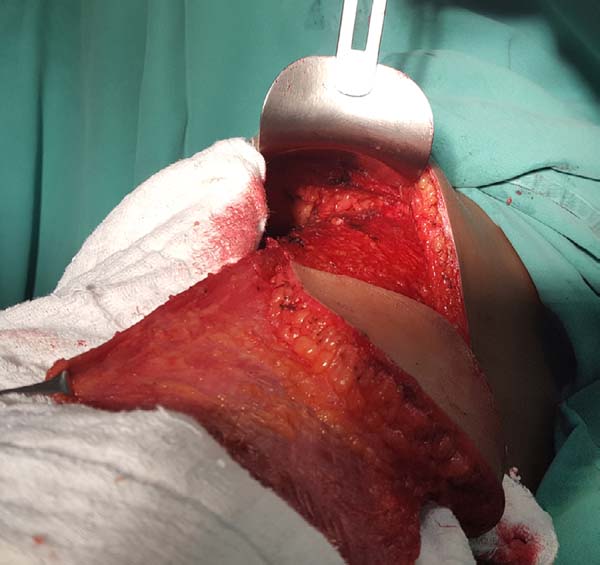
With the muscle dissected, a tunnel of approximately 6 cm width is created
through the axillary fascia, approximately at the height of the line of
deployment of the axillary hair (Figure 4). Taking care not to twist the muscle, the same is rotated to
the receptor site through the previous traction using the Allis forceps
(Figure 5).
Figura 4 - Dissected flap with preserved pedicle and infra-axillary
tunnel in the upper portion.
Figura 4 - Dissected flap with preserved pedicle and infra-axillary
tunnel in the upper portion.
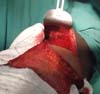
Figure 5 - Flap rotated to the receptor site and ready to be sutured;
preparation of a pocket for the silicone implant.
Figure 5 - Flap rotated to the receptor site and ready to be sutured;
preparation of a pocket for the silicone implant.
At this moment, the donor site can be synthesized with vicryl 2.0 adhesion
sutures and aspiration drainage with 4.8-gauge drains. The pocket for the
breast implant is created by attaching the muscle to the limits dissected
using nylon 2.0 sutures.
The implant is bathed in an antibiotic solution with cefazolin (1 g) and
gentamicin (80 mg) and then positioned in the pocket with a conclusion of
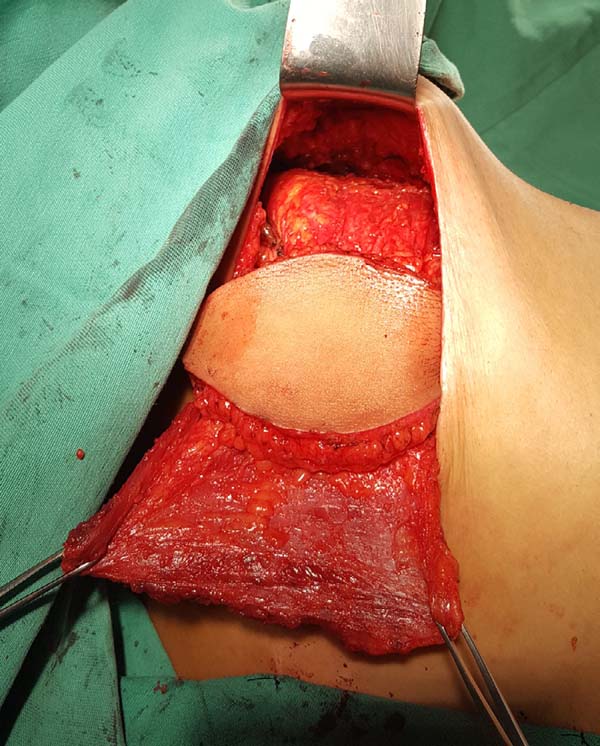
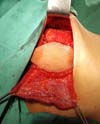
the suture. The skin island and the remaining breast skin envelope should be
customized so that the neobreast has a slight ptosis, which gives a more
natural result (Figure 6). The
recipient site should also be drained with a 6.4-gauge suction drain. The
drains are removed postoperatively when the daily flow is less than 40
mL.
Figura 6 - Immediate appearance after customization of the flap and
mastectomy skin.
Figura 6 - Immediate appearance after customization of the flap and
mastectomy skin.
RESULTS
A total of 29 patients underwent surgery during the study period, with a minimum
post-operative follow-up duration of 3 months. The mean age of the patients was
47.22 years, with the youngest patient was aged 28 years and the oldest, 76
years (Table 1).
Table 1 - Distribution of age and volume of breast implants.
| Variable |
Maximum |
Minimum |
Average |
| Age |
76 years |
28 years |
47.22 years |
| Breast Implant |
390 cc |
280 cc |
318 cc |
Table 1 - Distribution of age and volume of breast implants.
None of the patients underwent neoadjuvant chemotherapy. With respect to the
comorbidities detected, there were 3 patients with systemic hypertension and 1
patient with fibromyalgia (Table 2).
Table 2 - Distribution of comorbidities.
| Variable |
Absolute number |
Relative rate |
| Arterial hypertension |
3 |
10.5% |
| Fibromyalgia |
1 |
3.556% |
Table 2 - Distribution of comorbidities.
The timing of the reconstructive surgery was decided in conjunction with a team
of mastologists and included 26 immediate reconstructions and 3 late
reconstructions. There were no bilateral reconstructions. The implants used were
round format in 28 patients and anatomical in 1 patient. The volumes ranged from
260 cc to 390 cc, with an average of 318 cc. The average length of surgery was 1
h 45 m (Table 1).
The period of hospitalization was uniform with discharges on the second
postoperative day. The drains were removed when the daily flow fell below 40 mL,
with an average of 10 postoperative days but not exceeding 14 postoperative
days.
With respect to complications, there was one patient with a seroma in the
receptor site (3.5%), who also had a local infection without response to
antibiotic therapy, which resulted in extrusion of the breast implant.
One patient (3.5%) had partial necrosis of the skin island of the flap and local
cellulitis, with resolution after debridement, antibiotic therapy, and
dressings. The cause of necrosis can be attributed to the small cutaneous
segment detached from the muscle to better adapt to the receptor site defect.
Two patients (7.0%) suffered from the remnant cutaneous envelope of the breast
without major clinical repercussions. In these 3 patients, conservative measures
were sufficient since the mammary implant in this technique is completely
covered by the latissimus dorsi muscle and provides both protection in cases of
cutaneous necrosis and avoidance of external contamination.
One patient (3.5%) had extensive local recurrence and the implant was removed at
the request of the radiotherapy team according to a therapeutic rescue protocol.
One patient (3.5%), submitted to adjuvant radiotherapy, had a Baker III capsular
contracture after 18 months of breast reconstruction.
Four patients (14%) were submitted to scar reviews.
On clinical examination, one patient (3.5%) had a seroma in the dorsum that was
resolved with aspiration puncture. Imaging examinations were not requested
routinely to evaluate this condition.
Three patients (10.5%) displayed functional abduction limitations in the
articulation of the ipsilateral shoulder. One of these patients was diagnosed
with a winged scapula and forwarded to follow-up with a physiotherapist (Table 3).
Table 3 - Distribution of complications.
| Variable |
Absolute number |
Index on |
| Infection |
2 |
7.0% |
| Cutaneous necrosis |
3 |
10.5% |
| Implant extrusion |
1 |
3.5% |
| Removal of the implant |
1 |
3.5% |
| Seroma in receptor site |
1 |
3.5% |
| Seroma in donor site |
1 |
3.5% |
| Capsular contracture |
1 |
3.5% |
| Scar revision |
4 |
14% |
| Functional joint deficit |
3 |
10.5% |
Table 3 - Distribution of complications.
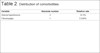
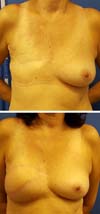
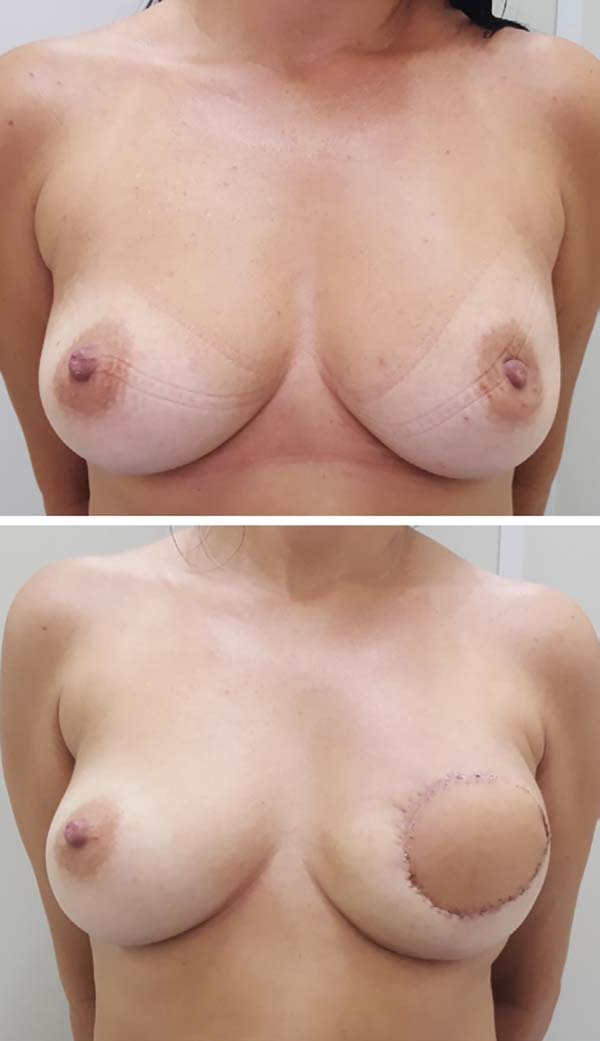
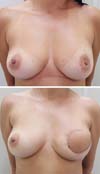
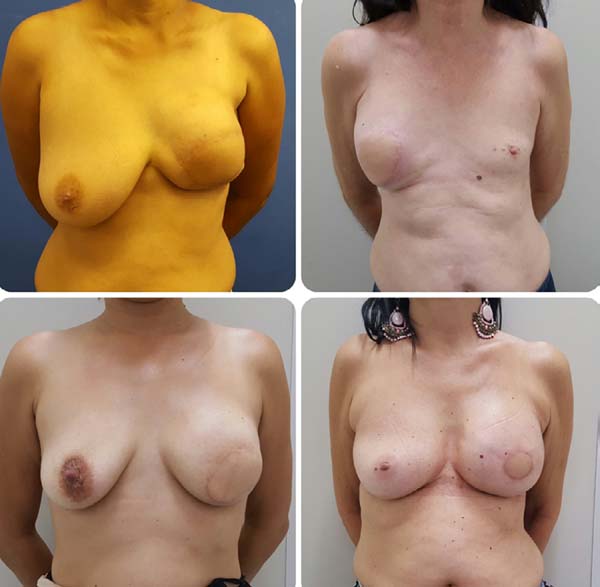
Postoperative results can be seen in Figures 7 to 9.
Figure 7 - Pre-operative and 6-month postoperative aspects. Late
reconstruction.
Figure 7 - Pre-operative and 6-month postoperative aspects. Late
reconstruction.
Figure 8 - Pre-operative and 3-month postoperative aspects. Immediate
reconstruction.
Figure 8 - Pre-operative and 3-month postoperative aspects. Immediate
reconstruction.
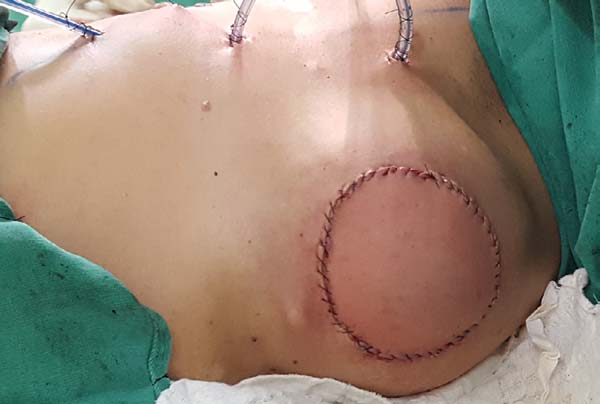
Figura 9 - Photographs of the patients in the sixth postoperative
month.
Figura 9 - Photographs of the patients in the sixth postoperative
month.
DISCUSSION
The surgical treatment of breast cancer has evolved over time. In 1894, Halstead
described the classical radical mastectomy as the first effective treatment for
breast cancer. In 1948 by comparative studies, Patey noted that the preservation
of the greater pectoral muscle does not compromise the local control of the
tumor, and this led to the term modified radical mastectomy.
To the extent that adjuvant systemic treatment has gained in importance,
conservative surgeries of the breast that preserve lymph nodes and segments of
the breast parenchyma became possible6,
leading to breast reconstruction efforts.
The LDMF was historically used to cover defects in the chest wall, either to
cover the defects arising from surgical breast excision or to treat sequelae
caused by radiotherapy. The advent of breast implants between the 1960s and
1970s helped to regain the lost breast volume when associated with the LDMF.
Techniques that rely exclusively on autologous tissues, such as the use of
transverse rectus abdominis muscle (TRAM), microsurgical flaps, and others that
use only alloplastic material positioned below the muscles of the anterior
thoracic region, have been described by surgeons who aimed for alternatives that
could be adapted for each patient.
The LDMF not only weathered time but also gained popularity and is now a mainstay
of a large number of plastic surgeons. There are a few anatomical variations of
the LDMF, which has reliable caliber pedicle and wide muscle width, which allows
full coverage of implants, including the bulky implants. Furthermore, morbidity
in the donor area is small, such as a functional deficit in the articulation of
the shoulder, seroma formation, or persistent back pain.
The applicability LDMF is vast, and the LDMF may be used for coverage in cases of
thin coverage of soft parts in the breast region, irradiated anterior chest
wall, previous resection of the pectoralis major, ptotic or small to moderate
size contralateral breast, previous unsuccessful reconstruction with exclusive
breast implant, previous surgeries in the abdomen, and lack of experience in
microsurgical techniques.
The skin island on the dorsum can be ample; however, the primary closure width
can be 9 cm with a length of 18 cm, but 17 × 7 cm flaps are suitable. Fat
compartments in the back were described in the 1990s by Delay et al.7, with the aim of incorporating a greater
quantity of fatty fascia in the flap and reconstructing small to medium sized
breasts without the need for implants. However, contour defects and seroma
formation in the donor area decreased interest in this technique8.
Moderate amounts of fat fascia can be mobilized to the LDMF for improving both
the consistency and outline of the neobreast, allowing for a smoother and
less-marked transition in its upper pole, as described by Tavares-Filho et
al.9. New generations of implants with
high-cohesive gel confer greater stability to the breast shape and better
consistency on touch, resulting in good esthetic results in reconstructions
performed with the LDMF, even without an excessive fatty fascia harvest10.
Breast reconstruction with the LDMF can be both delayed and immediate (at the
same time as the mastectomy). The decubitus for this surgery can vary. For
surgeons who choose to harvest the LDMF in a ventral decubitus, there is a need
to change the position of the patient at least once in cases of late
reconstruction or even twice in cases of immediate reconstruction.
When opting for a lateral decubitus, the position change occurs only once in
immediate reconstructions and becomes unnecessary in late reconstructions.
Changes in the decubitus position carry the risk of joint, ligament, or even
nerve lesions. In addition, they require more surgical material (new sterile
fields) and an increase in the total length of surgery with all its potential
morbid (infection, thromboembolic events, hypothermia, etc.) and financial
implications (surgical room time and anesthetic medications). Specific studies
are needed to quantify these variables.
The systematization of the surgical technique allows the surgeon to follow a
certain sequence, thus, minimizing the loss of time. Systematization begins with
the orientation and training of nursing assistants who will position the
patient, making this dynamic moment brief and safe. The surgical procedure is
then performed by following the described technique step-by-step; this ensures
that the assistant surgeon and the scrub nurse know the exact sequence of
presentation and the instruments used.
A disadvantage may be that some surgeons may take time to familiarize themselves
with the dissection in a position different from the usual dorsal decubitus, in
addition to difficulty in properly customizing the skin island and the remaining
breast skin envelope, which pend slightly medially. The end result can be a
satisfactory neobreast volume, but with excessive ptosis and/or markedly
eccentric positioning of the skin island.
Literature shows that the rate of complications for this surgery is low. A seroma
at the donor site is a fairly common complication with studies showing
complication rates that vary from 16% to 79%11. The association of adhesion sutures and aspiration drains may
account for the low seroma rate observed, which corroborates with the data
presented by Cammarota et al. 201612.
Other complications are described with lower rates of incidence: skin infections
account for 3.3% of the complications; flap necrosis, 1.3%; operative wound
dehiscence, 0.6%; and clinical complications, 3.2%12. A larger sample set would show better approximation of
the rate of complications observed with those previously reported.
The rates of capsular contracture are variable and range from 6% to 68%13,14. Longer follow-up duration is necessary to assess the
real contracture rate. Adjuvant radiotherapy may be associated with an increase
in the contracture rate resulting in actinic capsular contracture15.
An alternative for patients requiring adjuvant radiotherapy is the association of
LDMF with a breast tissue expander. A silicone implant would be used only occur
after complete tissue expansion and completion of radiotherapy, when it is
possible to adjust the format of the neobreast and correct the possible
contracture stigmas by capsulotomy or capsulectomy16,17.
Plastic surgery options have evolved; therefore, fewer morbidities are associated
with these patients. The use of microsurgical flaps or implants associated with
dermal matrices has been reported in recent years in specialized publications as
alternatives that promote low morbidity and good results4,18.
However, the use of microsurgical flaps requires specific prolonged surgical
training, and dermal matrices are still not feasible in Brazil due to the high
associated costs. Consequently, the LDMF in combination with silicone implants
continues to be an excellent option for plastic surgeons.
CONCLUSION
The systematization of breast reconstruction using the LDMF combined with
silicone breast implants in the lateral decubitus position is a safe alternative
for the plastic surgeon, which is a rapid procedure with consistent results.
These advantages can be availed when the medical professionals are familiar with
the surgery in this patient positioning.
COLLABORATIONS
|
ICGL
|
Analysis and/or interpretation of data; conception and design of the
study; completion of surgeries and/or experiments.
|
REFERENCES
1. American Cancer Society. How Common Is Breast Cancer? [acesso 2017
Jun 1]. Disponível em: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
2. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José
Alencar Gomes da Silva (INCA). Câncer de Mama. Estimativa de novos casos.
[acesso 2017 Jun 1]. Disponível em: http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/mama/cancer_mama++
3. Secretaria de Comunicação Social do Senado Federal. Lei Garante
Reconstrução da Mama em Seguida à Retirada de Câncer. [acesso 2017 Jun 1].
Disponível em: http://www12.senado.leg.br/noticias/materias/2013/05/07/lei-garante-reconstrucao-da-mama-em-seguida-a-retirada-de-cancer
4. Macadam SA, Bovill ES, Buchel EW, Lennox PA. Evidence-Based
Medicine: Autologous Breast Reconstruction. Plast Reconstr Surg.
2017;139(1):204e-29e. DOI: http://dx.doi.org/10.1097/PRS.0000000000002855
5. Wei FC, Mardini S. Retalhos e cirurgia plástica reconstrutora. Rio
de Janeiro: Di Livros; 2012. p. 343-64.
6. Gabka CJ, Bohmert H. Cirurgia plástica e reconstrutiva da mama. 2ª
ed. Porto Alegre: Artmed; 2010.
7. Delay E, Gounot N, Bouillot A, Zlatoff P, Rivoire M. Autologous
latissimus breast reconstruction: a 3-year clinical experience with 100
patients. Plast Reconstr Surg. 1998;102(5):1461-78. PMID: 9774000 DOI: http://dx.doi.org/10.1097/00006534-199810000-00020
8. Heitmann C, Pelzer M, Kuentscher M, Menke H, Germann G. The extended
latissimus dorsi flap revisited. Plast Reconstr Surg. 2003;111(5):1697-701.
PMID: 12655217 DOI: http://dx.doi.org/10.1097/01.PRS.0000055444.84307.75
9. Tavares-Filho JM, Franco D, Moreto L, Porchat C, Franco T.
Utilização do retalho miocutâneo de grande dorsal, com extensão adiposa, nas
reconstruções mamárias: uma opção para preenchimento do polo superior. Rev Bras
Cir Plást. 2015;30(3):423-8.
10. D'Alessandro GS, Povedano A, Santos LKIL, Santos RA, Góes JCS.
Reconstrução mamária imediata com retalho do músculo grande dorsal e implante de
silicone. Rev Bras Cir Plást. 2015;30(2):163-71.
11. Roy MK, Shrotia S, Holcombe C, Webster DJ, Hughes LE, Mansel RE.
Complications of latissimus dorsi myocutaneous flap breast reconstruction. Eur J
Surg Oncol. 1998;24(3):162-5. PMID: 9630851 DOI: http://dx.doi.org/10.1016/S0748-7983(98)92810-4
12. Cammarota MC, Ribeiro Junior I, Lima RQ, Almeida CM, Moura LG, Daher
LMC, et al. Estudo do uso de pontos de adesão para minimizar a formação de
seroma após mastectomia com reconstrução imediata. Rev Bras Cir Plást.
2016;31(2):158-65.
13. Gart MS, Smetona JT, Hanwright PJ, Fine NA, Bethke KP, Khan SA, et
al. Autologous options for postmastectomy breast reconstruction: a comparison of
outcomes based on the American College of Surgeons National Surgical Quality
Improvement Program. J Am Coll Surg. 2013;216(2):229-38.
14. Perdikis G, Koonce S, Collis G, Eck D. Latissimus dorsi myocutaneous
flap for breast reconstruction: bad rap or good flap? Eplasty.
2011;11e39.
15. McCarthy CM, Pusic AL, Disa JJ, McCormick BL, Montgomery LL,
Cordeiro PG. Unilateral postoperative chest wall radiotherapy in bilateral
tissue expander/implant reconstruction patients: a prospective outcomes
analysis. Plast Reconstr Surg. 2005;116(6):1642-7. PMID:
16267426
16. Losken A, Nicholas CS, Pineel XA, Carlson GW. Outcomes evaluation
following breast reconstruction using latissimus dorsi myocutaneous flaps. Ann
Plast Surg. 2010;65(1):17-22.
17. Lennox PA, Bovill ES, Macadam AS. Evidence-Based Medicine:
Alloplastic Breast Reconstruction. Plast Reconstr Surg.
2017;140(1):94e-108e.
18. Qureshi AA, Broderick KP, Belz J, Funk S, Reaven N, Brandt KE, et
al. Uneventful versus Successful Reconstruction and Outcome Pathways in
Implant-Based Breast Reconstruction with Acellular Dermal Matrices. Plast
Reconstr Surg. 2016;138(2):173e- 83e.
1. Hospital Napoleão Laureano, João Pessoa, PB,
Brazil
2. Hospital das Clínicas, Recife, PE,
Brazil.
3. Sociedade Brasileira de Cirurgia Plástica, São
Paulo, SP, Brazil.
Corresponding author: Igor Chaves Gomes
Luna, Rua Reinaldo Tavares de Melo, 142 - Manaíra - João Pessoa, PB,
Brazil. Zip Code 58038-300. E-mail:
igorluna_med@hotmail.com
Article received: June 10, 2017.
Article accepted: September 18, 2017.
Conflicts of interest: none.