INTRODUCTION
According to the International Society of Aesthetic Plastic
Surgery, Brazil ranks second globally for aesthetic surgical
procedures. The implantation of a silicone breast enlargement prosthesis remains
the most popular surgical procedure worldwide (15.8%), followed by liposuction
(14%), blepharoplasty (12.9%), rhinoplasty (7.6%), and abdominoplasty
(7.4%)1.
Of these surgical procedures, abdominoplasty and liposuction entail major
intercurrences and complications2-4.
Fibrosis, intense edema, and ecchymosis are complications that greatly challenge
the dermatofunctional physiotherapist, who, over the past few years, has sought
effective treatments to act in the pre-, trans-, and postoperative periods,
demonstrating the importance of this healthcare professional5.
The physiotherapist’s role during the preoperative period remains very
restricted6. Lange7 reports the use of cosmetics,
nutricosmetics, and low glycemic index diets to improve the healing process,
rearrange collagen, and reduce the rates of fibrosis, intense edema, and
ecchymosis formation.
The physiotherapeutic approach in the transoperative period of plastic surgery
has not been well demonstrated. Lange7
mentioned only the use of Unna boots and compression stockings for the
prevention of deep venous thrombosis.
Thus, the present study proposes a new approach to the preoperative (use of
antiglycans, nutricosmetics and nutritional orientations), transoperative
(lymphatic taping and containment foam), and postoperative (manual lymphatic
drainage, microcurrent, red light-emitting diode [LED], and taping) management
with the goal of preventing and minimizing fibrosis, severe edema, and
ecchymosis, accelerating patient recovery, and reducing the number of required
physiotherapy sessions.
OBJECTIVE
The objective of this study is to evaluate the occurrence of postoperative
ecchymosis, edema, and fibrosis in patients undergoing liposuction and/or
abdominoplasty and statistically correlate these occurrences with the pre- and
transoperative treatment.
METHOD
This controlled clinical trial was performed from June to December 2016. The
study received approval from the Ethics Committee of the Center of Higher
Education of Campos Gerais, Ponta Grossa, PR (55410316.0.0000.5215).
Female subjects in the preoperative phase of abdominal plastic surgery who were
aged 18-56 years, had the surgical indication of abdominoplasty and/or abdominal
liposuction, and were scheduled at least 7 days before surgery were admitted to
the study. The sample consisted of 20 patients divided evenly into the control
group (CG) and the experimental group (EG).
Patients in the CG and EG were evaluated preoperatively and daily after the
4th postoperative day until the end of treatment.
The 20 patients were evaluated preoperatively, and personal details, surgical
details, abdominal semiology, anthropometric measurements, and
photodocumentation were collected.
The postoperative treatment in the CG was started from the 4th day for
1 hour each session for the first 3 sequential sessions, and the remaining
alternated 3 times in the first week, 3 times in the second week, twice in the
third week and once in the last 4 weeks for a total of 15 sessions.
The therapeutic resources used in all sessions were: manual lymphatic drainage
with the Leduc method in the lower limbs, upper limbs, abdomen and flanks,
microcurrent (frequency, 250 Hz; intensity, 150 µA) for 20 minutes in the
abdomen, red LED (650-959 nm) for 20 minutes in the abdomen, and application of
taping in the operated area with the application chosen in accordance with the
alteration found (edema, ecchymosis, or fibrosis), with a “web” or “basket” cut
for fibrosis, “fan” or “octopus” for edema, and “hashtag” cut for ecchymosis,
with a rest of 3-5 days between them and 1 day to the next application when
necessary.
The EG group received care during the pre-, trans-, and postoperative periods.
Preoperatively, guidelines regarding postoperative care, nutritional guidance
with low glucose ingestion, a nutricosmetic oral antiglycant
(Exsynutriment®, Glycoxil®, and Bioarct®,
100 mg each, 30 capsules, 1 capsule per day), and a topical antiglycant
(Alistin® 10%, 30 g, 2 times a day in the area subjected to
surgery), were provided and indicated to be used for 30 days or until the
product supply was exhausted.
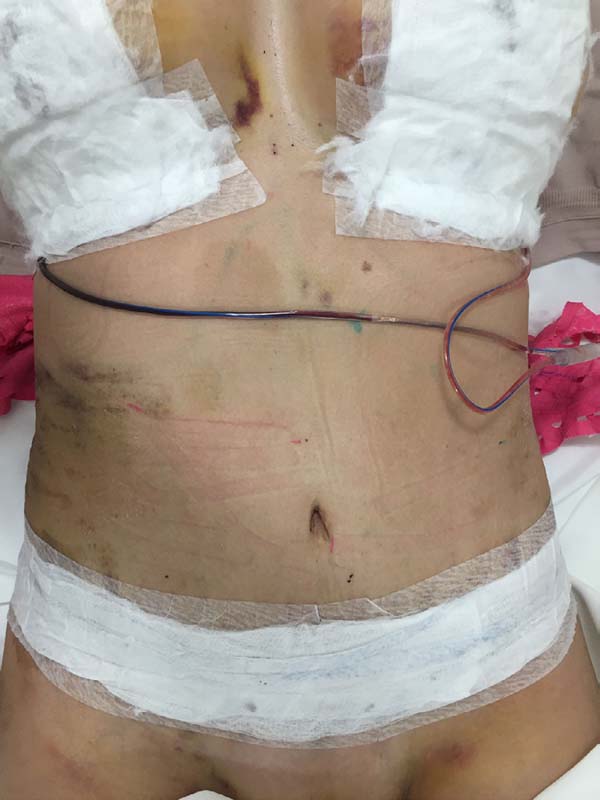
The intraoperative treatment of the EG group was performed with the application
of lymphatic taping in a “fan” or “octopus” form in the operated regions. The
dressings were cut into five different portions, positioned with minimum tension
(0-15%) in the regions of the abdomen (with a fixed base in the axillary region
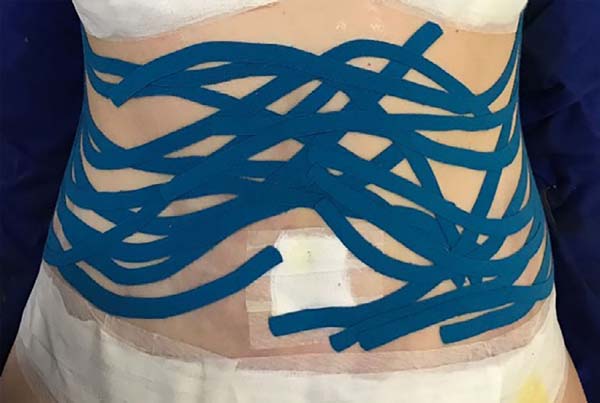
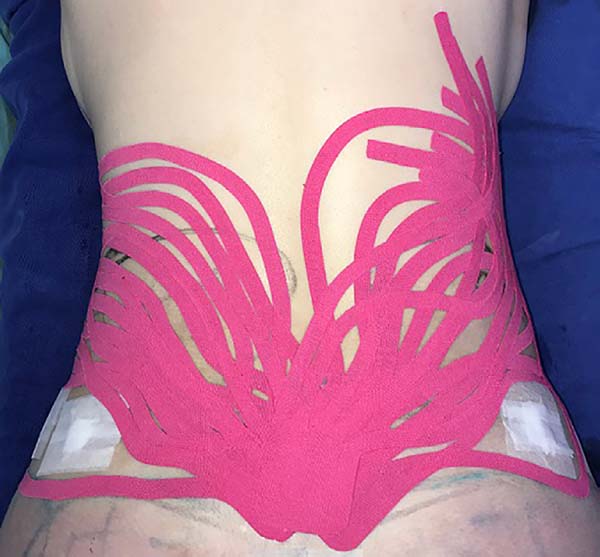
bilaterally) (Figure 1) and flanks (with a
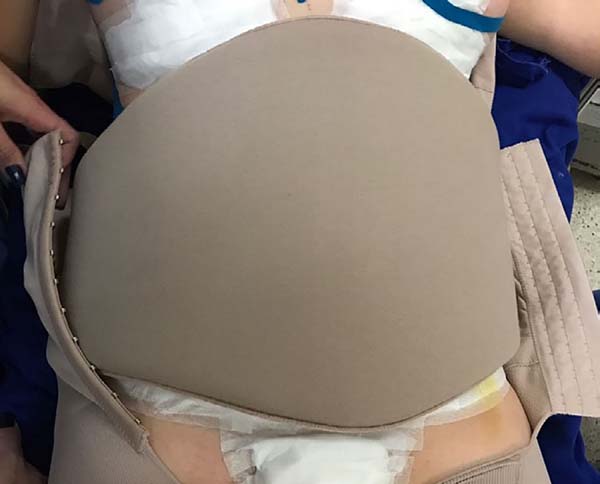
fixed base in the coccygeal region) (Figure 2) and contention foam 360° in the operated region under the surgical
mesh (Figure 3). The same postoperative
treatment was offered to patients in the EG and CG.
Figure 1 - Fan taping on the anterior abdomen.
Figure 1 - Fan taping on the anterior abdomen.
Figure 2 - Fan taping on the flanks.
Figure 2 - Fan taping on the flanks.
Figure 3 - Containment foam under the surgical mesh.
Figure 3 - Containment foam under the surgical mesh.
Fibrosis formation was evaluated during all care sessions through palpation,
visual inspection, contact thermography analysis, and photodocumentation in both
groups. Edema was analyzed based on perimetry and body weight in both groups,
while ecchymosis was evaluated using photodocumentation in both groups.
A database was created of the collected data that were subjected to analysis.
Descriptive statistics and analysis of variance were the statistical methods
used.
The following data were statistically analyzed in both groups: number of
sessions; start of fibrosis; resolution of fibrosis; resolution of ecchymosis;
perimetry of the iliac crest, inframammary groove, and navel; initial
thermography; degree of fibrosis; ecchymosis type; and pain.
RESULTS
With the data collected during the preoperative and postoperative care of
patients in the CG and EG, the study variables were assessed and compared.
The data of the quantitative variables were subjected to the
Kolmogorov-Smirnov (KS) normality test to
guide the choice between parametric or non-parametric tests.
The standard deviation of the variables by groups approved in the normality tests
were subjected to the T test to guide the choice of the most suitable test for
parametric analysis of variance of the means test (T test or T test with Welch
correction). The data sets not approved in the KS test were subjected to the
Mann-Whitney non-parametric test.
The p values of the analysis of variance and summary statistics
of the numerical variables are presented in Table 1.
Table 1 - Summary statistic and p value of the analysis of variance between the
groups.
| Variable |
|
|
|
|
Group |
P value
|
| |
|
|
|
Control |
Experimental |
| Age
(years)
|
|
|
|
µ |
32.1 |
39.9 |
0.1251 |
| |
|
|
± |
10.2 |
11.5 |
| No.
of sessions
|
|
|
|
µ |
23.1 |
14.6 |
*0.0032 |
| |
|
|
± |
6.7 |
0.7 |
| Beginning of fibrosis |
|
|
|
µ |
19 |
3.4 |
*0.0019 |
| |
|
|
± |
4.7 |
7.2 |
| Resolution of fibrosis |
|
|
|
µ |
48.6 |
11.7 |
*0.0058 |
| |
|
|
± |
22.2 |
16.3 |
| Rest
time
|
|
|
|
µ |
15 |
14.5 |
0.4704 |
| |
|
|
± |
0 |
1.6 |
| Resolution ecchymosis |
|
|
µ |
17.6 |
7.8 |
*0.0002 |
| |
|
± |
5 |
4.3 |
| Weight (kg) |
PO |
|
|
µ |
70.97 |
69.49 |
0.7071 |
| |
± |
7.76 |
9.49 |
| 4th PO
|
|
|
µ |
70.5 |
69.75 |
0.8177 |
| |
± |
7.06 |
9.92 |
| Final |
|
|
µ |
66.51 |
65.66 |
0.8177 |
| |
± |
7.58 |
8.63 |
| Variation (from the PO) |
|
4th PO |
µ |
20.92 |
23.6 |
0.3073 |
| ± |
12.52 |
16.36 |
| Final |
µ |
-20.92 |
-23.6 |
0.6852 |
| ± |
12.52 |
16.36 |
| Perimetry (cm) |
Pré-operatória |
|
Inframammary groove |
µ |
85.5 |
84 |
0.5601 |
| ± |
5.8 |
5.5 |
| Navel |
µ |
92.9 |
91.9 |
0.78 |
| ± |
8 |
7.8 |
| Iliac
crest
|
µ |
97.3 |
97.5 |
0.9379 |
| ± |
5.2 |
6.1 |
| 4th PO
|
|
Inframammary groove |
µ |
88.1 |
81.7 |
*0.0261 |
| ± |
5.4 |
6.4 |
| Navel |
µ |
95.1 |
88.6 |
0.0567 |
| ± |
8 |
6.2 |
| Iliac
crest
|
µ |
99.8 |
94.7 |
*0.0303 |
| ± |
5.2 |
4.5 |
| Final |
|
Inframammary groove |
µ |
81 |
77.3 |
0.1518 |
| ± |
5.4 |
5.7 |
| Navel |
µ |
86 |
81 |
0.133 |
| ± |
8.2 |
5.7 |
| Iliac
crest
|
µ |
89.3 |
88.5 |
0.7447 |
| ± |
5.7 |
5.1 |
| Variation (from the PO) |
4th PO
|
Inframammary groove |
µ |
3.09 |
2.78 |
*0.0001 |
| ± |
1.15 |
2.29 |
| Navel |
µ |
2.38 |
3.42 |
*0.0022 |
| ± |
0.49 |
4.3 |
| Iliac
crest
|
µ |
2.58 |
-2.76 |
*0.0002 |
| ± |
1.08 |
2.84 |
| Final |
Inframammary groove |
µ |
-5.09 |
-8.25 |
*0.0027
|
| ± |
1.75 |
2.28 |
| Navel |
µ |
-7.31 |
-12.26 |
*0.0133
|
| ± |
1.48 |
5 |
| Iliac crest |
µ |
-8.04 |
-9.48 |
0.1821 |
| ± |
1.71 |
2.8 |
Table 1 - Summary statistic and p value of the analysis of variance between the
groups.
The variables that presented significant differences between the means
(p < 0.05) included number of sessions, beginning of
fibrosis, resolution of the fibrosis, resolution of the ecchymosis, perimetry on
the 4th postoperative day of the iliac crest and the inframammary groove, and
perimetry of the 4th postoperative day and final assessment of the inframammary
groove and navel.
From the T test with Welch correction, significant (p < 0.05)
differences were found between the EG and the CG with regard to the number of
sessions; therefore, we rejected the null hypothesis.
The EG (μ =14.60 ± 0.70) presented a statistically significant lower mean number
of sessions (p = 0.0032) than the CG (μ = 23.10 ± 6.71).
No pain was reported in the operated region in the EG, while the CG reported pain
a mean 5.50 ± 1.58 postoperative days.
Perimetry was performed preoperatively, on the 4th postoperative day,
and at the end of the treatment in the inframammary groove, navel, and iliac
crest.
On the 4th postoperative day, significant differences
(p < 0.05) were observed in the inframammary groove and
iliac crest. The EG displayed a larger mean reduction in the perimetry than the
CG in all different periods and anatomic parts except the iliac crest.
Significant differences (p < 0.05) were found between the EG
and CG in relation to the resolution of fibrosis (Table 2); thus, we rejected the null hypothesis.
Table 2 - Summary statistic and p value of the analysis of variance of
resolution of fibrosis between the groups.
| Variable |
|
Group |
P value
|
| |
Control |
Experimental |
| Resolution of fibrosis |
µ |
48.6 |
11.7 |
0.0058 |
| ± |
22.2 |
16.3 |
Table 2 - Summary statistic and p value of the analysis of variance of
resolution of fibrosis between the groups.
The EG displayed a statically significant (p = 0.0058) lower
mean resolution of fibrosis (μ = 11.7 ± 16.3) than the CG (μ = 48.6 ± 22.2).
From the non-paired T test, there were significant differences (p < 0.05)
between the EG and CG in resolution of ecchymosis (Table 3); thus, we rejected the null hypothesis.
Table 3 - Summary statistic and p value of the analysis of variance of the
resolution of ecchymosis between groups.
| Variable |
|
Group |
P value
|
| |
Control |
Experimental |
| Resolution of ecchymosis |
µ |
17.6 |
7.8 |
0.0002 |
| ± |
5.0 |
4.3 |
Table 3 - Summary statistic and p value of the analysis of variance of the
resolution of ecchymosis between groups.
The EG displayed a statically significant (p = 0.0002) lower
mean resolution of ecchymosis (µ = 7.8 ± 4.3) than the CG (μ = 17.6 ± 5.0).
p values < 0.05 indicate that the deviations are significant,
that the variables are dependent, and that the samples differ significantly
regarding the proportions of these classes; therefore, we rejected the null
hypothesis (Table 4).
Table 4 - Summary statistic and p value of the analysis of variance between the
groups.
| Variable |
Classes |
Controle |
Experimental |
p valor
|
| Fibrosis |
No |
0 |
8 |
*0.0003 |
| Yes |
10 |
2 |
| Initial fibrosis degree |
0 |
0 |
8 |
*0.0002 |
| I |
0 |
2 |
| II |
6 |
0 |
| III |
4 |
0 |
| Initial thermography |
I |
0 |
2 |
*0.0002 |
| II |
6 |
0 |
| III |
4 |
0 |
| Normal |
0 |
8 |
| Intense edema |
No |
5 |
10 |
*0.0325 |
| Yes |
5 |
0 |
| Suggillation ecchymosis |
Abdomen and flanks |
2 |
0 |
*0.0056 |
| Lower abdomen |
0 |
1 |
| R and L lateral abdomen |
2 |
0 |
| Right flank |
0 |
1 |
| Flanks |
6 |
0 |
| R and L flanks |
0 |
1 |
| Lower lumbar |
0 |
1 |
| No |
0 |
6 |
Table 4 - Summary statistic and p value of the analysis of variance between the
groups.
The results indicate that:
The occurrence of fibrosis in the EG was significantly
(p = 0.0003) less common than in the CG when all
elements were presented;
The degree of fibrosis was lower in the EG (p =
0.0002);
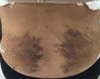
Initial thermography (Figures 4 and
5) was predominantly normal in
the EG (p = 0.0002);
Figure 4 - Thermography image of a patient in the control
group.
Figure 4 - Thermography image of a patient in the control
group.
Figure 5 - Thermography image of a patient in the experimental
group.
Figure 5 - Thermography image of a patient in the experimental
group.
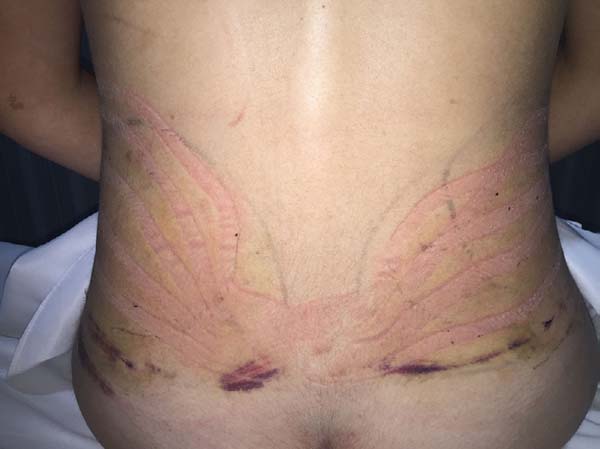
Non-occurrence of intense edema in the EG (p = 0.0325)
as shown in Figures 6 and 7;
Figure 6 - Transoperative aspect of a patient in the experimental
group.
Figure 6 - Transoperative aspect of a patient in the experimental
group.
Figure 7 - 4th postoperative day image of a patient in
the experimental group.
Figure 7 - 4th postoperative day image of a patient in
the experimental group.
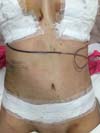
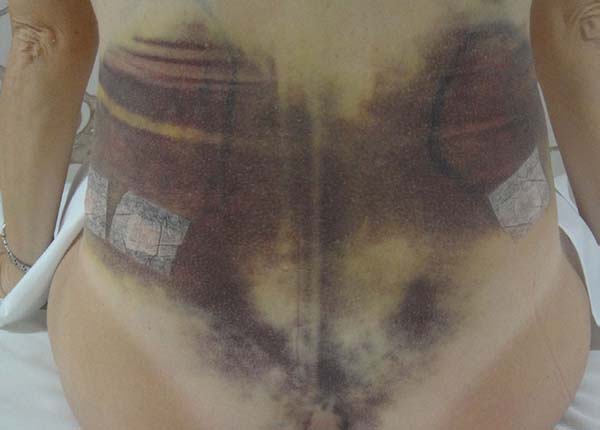
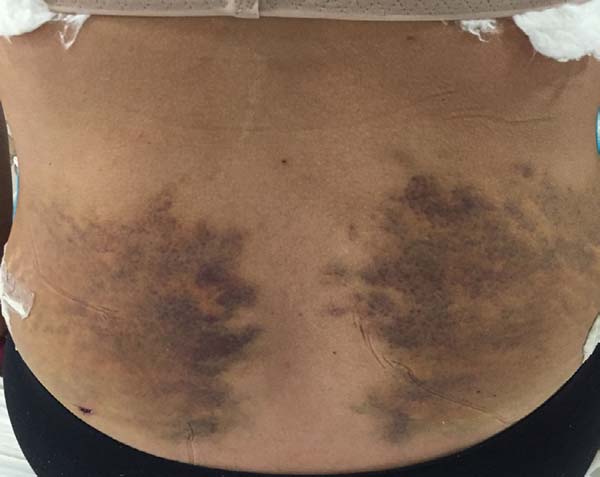
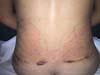
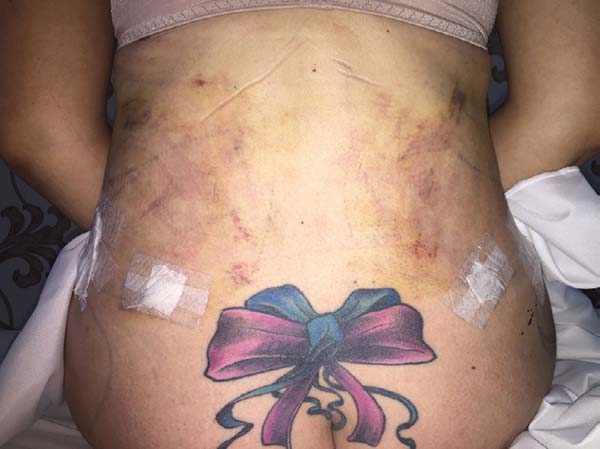
Non-occurrence of ecchymosis was higher in the EG (p =
0.0056) as shown in Figures 8 and
9;
Figure 8 - 4th postoperative day image of a patient in
the control group.
Figure 8 - 4th postoperative day image of a patient in
the control group.
Figure 9 - 4th postoperative day image of a patient in
the experimental group.
Figure 9 - 4th postoperative day image of a patient in
the experimental group.
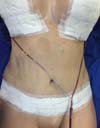
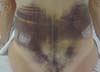
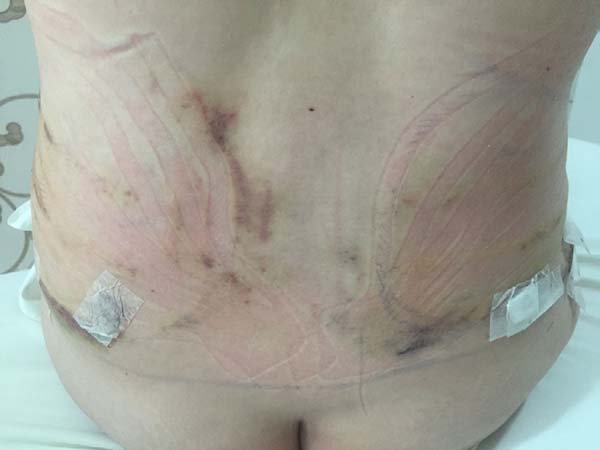
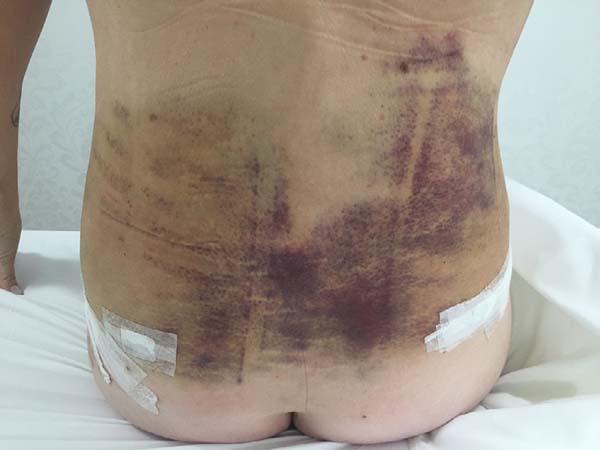
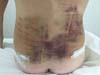
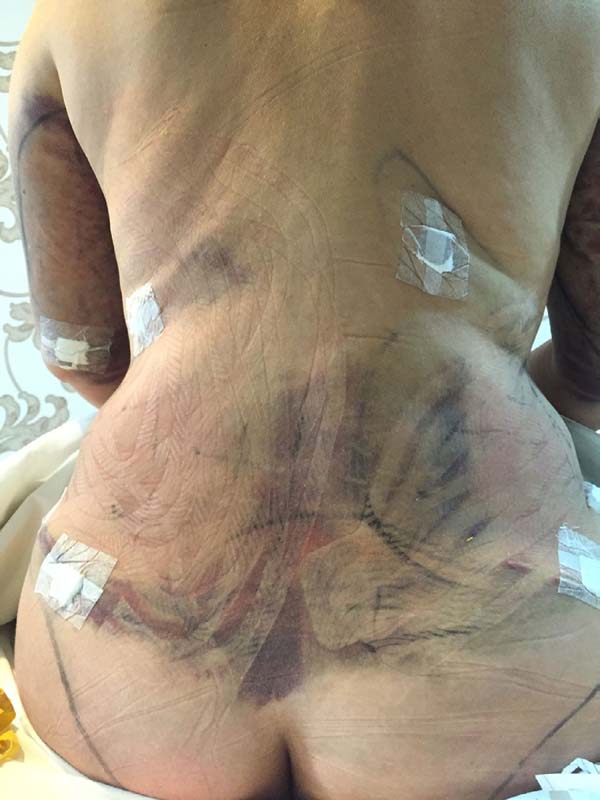
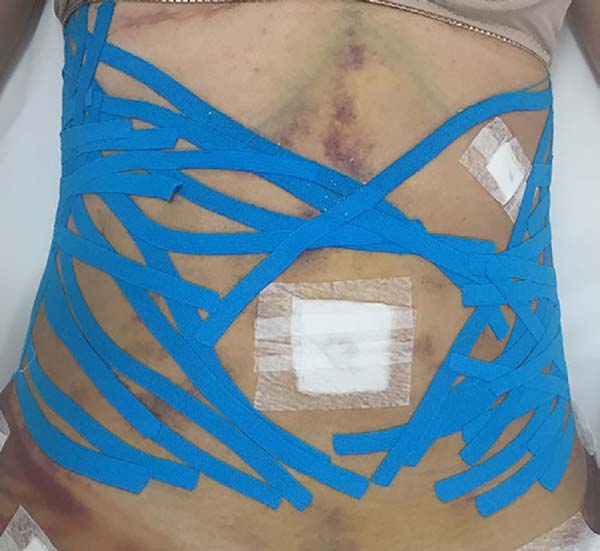
Non-occurrence of ecchymosis in the flanks; abdomen and flanks; and
lateral abdomen in the same proportions as in the CG (p
= 0.0056) (Figures 10-13).
Figure 10 - 4th postoperative day image of a patient in
the control group.
Figure 10 - 4th postoperative day image of a patient in
the control group.
Figure 11 - 4th postoperative day image of a patient in
the experimental group.
Figure 11 - 4th postoperative day image of a patient in
the experimental group.
Figure 12 - 4th postoperative day image of a patient in
the control group.
Figure 12 - 4th postoperative day image of a patient in
the control group.
Figure 13 - 4th postoperative day image of a patient in
the experimental group.
Figure 13 - 4th postoperative day image of a patient in
the experimental group.
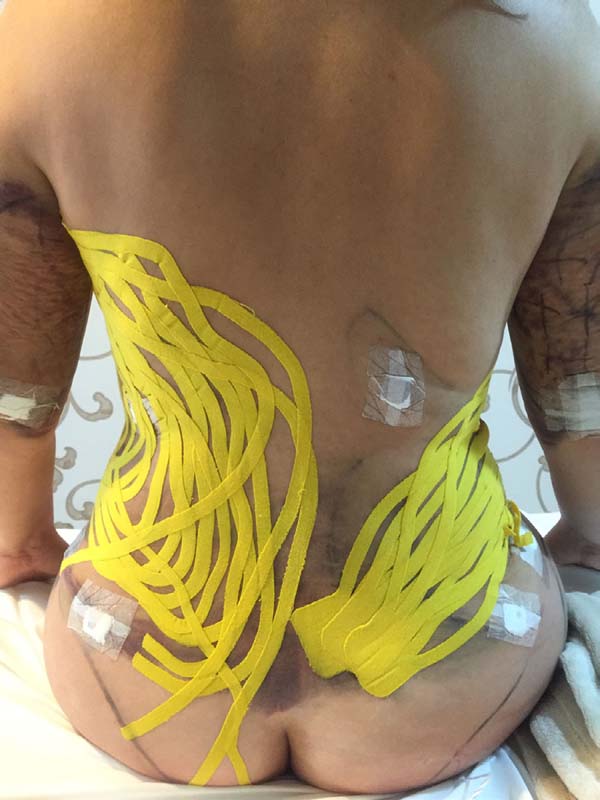
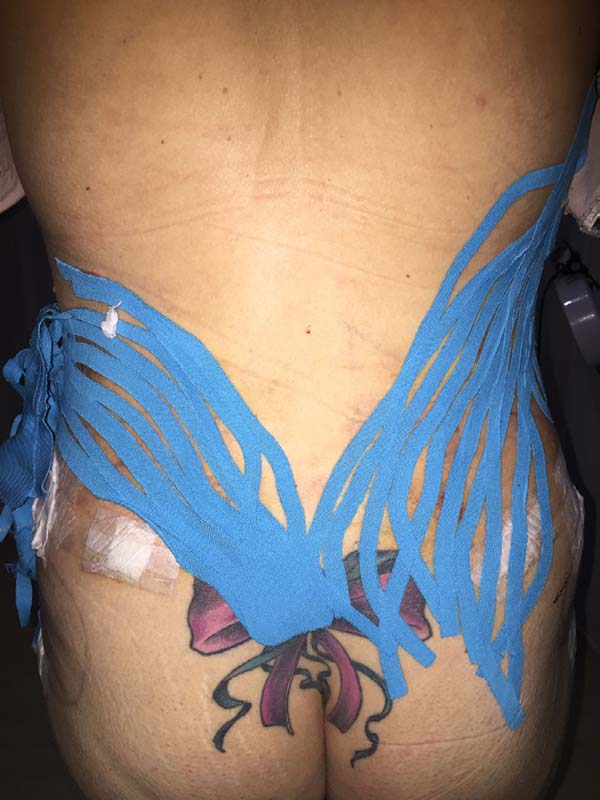
Moreover, in two patients in the EG, a difference was verified between the
placement of the base of the taping compared to the axillary lymph node and
coccygeal lymph node. There was no visual difference between the two positions
in the two patients (Figures 14-17).
Figure 14 - Taping in the left axillary base and right coccygeal
base.
Figure 14 - Taping in the left axillary base and right coccygeal
base.
Figure 15 - Result of taping application.
Figure 15 - Result of taping application.
Figure 16 - Taping in the right axillary base and left coccygeal
base.
Figure 16 - Taping in the right axillary base and left coccygeal
base.
Figure 17 - Result of taping application.
Figure 17 - Result of taping application.
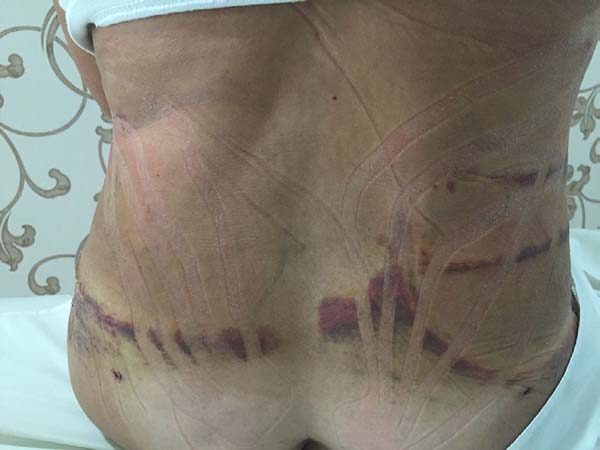
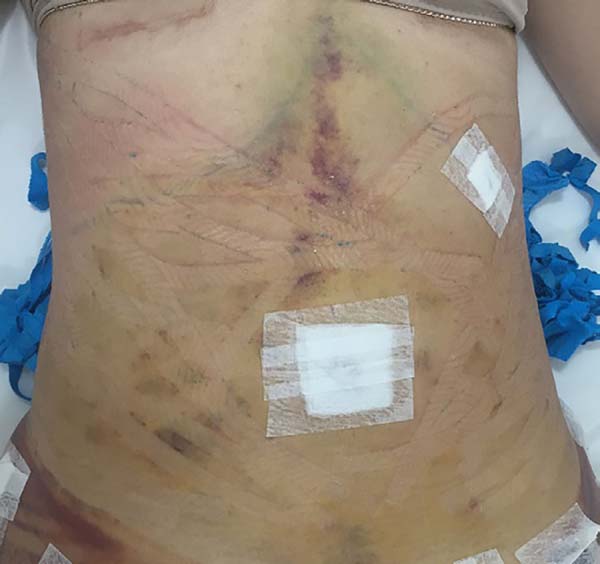
In regions in which taping was not used, a greater degree of visible ecchymosis
was visible (Figures 18 and 19).
Figure 18 - Placing of taping on the abdomen.
Figure 18 - Placing of taping on the abdomen.
Figure 19 - Visual analysis of ecchymosis formation on the abdomen.
Figure 19 - Visual analysis of ecchymosis formation on the abdomen.
DISCUSSION
The variables of the EG that presented significant differences were number of
sessions, beginning of fibrosis, resolution of fibrosis, resolution of
ecchymosis, and perimetry on the 4th postoperative day.
The mean number of sessions was 14.6 in the EG and 23.1 in the CG, showing that
the approach in the pre-, trans-, and postoperative periods reduces the number
of sessions.
Fibrosis formation in the EG occurred in only 2 patients on the 18th
and 16th postoperative day; in contrast, in the CG, all patients
developed fibrosis on the 19th postoperative day. According to
Lange7, Schwartz8, and Lange & Chi9, we usually palpate and/or visualize fibrosis after the first week
due to the collagen synthesis being more intense between the 6th and
17th days. This corroborates the results of the EG in that 80% of
those patients did not present fibrosis, as most did not present ecchymosis on
the 4th postoperative day.
The resolution of fibrosis of the two patients in the EG occurred on the
45th and 40th postoperative days compared to 48.6 days
in the CG. In this study, the preoperative period along with the lymphatic
taping and containment foam transoperatively promoted improved metabolism due to
constant lymphatic drainage.
Chi et al.10 showed results of lymphatic
taping accompanied by manual lymphatic drainage in the postoperative period, in
which they obtained a total reversal of fibrosis in patients who were in the
proliferative phase suggesting the use of transoperative lymphatic taping to
prevent fibrosis.
The EG obtained significant results (p = 0.0002) in the
resolution of ecchymosis compared to the CG. This finding corroborates with
those of Zanchet & Del Vecchio11, who
found absorption of ecchymosis with application of taping.
Significant differences (p < 0.05) were observed on the
4th postoperative day in the inframammary groove and iliac crest,
where we observed the largest perimetry in the CG compared to the EG. This shows
that the preoperative period together with the use of the lymphatic taping and
foam containment effectively reduced edema. There is no scientific evidence of
this approach (pre-, trans-, and postoperative) in plastic surgery that would
enable a comparison with our results.
For the treatment of edema, some studies are already been published, such as the
case study of Chou et al.12 showing the
effects of taping in a patient with lymphedema secondary to breast cancer.
According to Bosman & Piller13, the
application of taping favors the process of expansion of the initial lymphatic
vessels, allowing the liquid present in the interstitium to be absorbed by the
lymphatic network.
According to Van Zuilen et al.14, Neves et
al.15, and Bosman & Piller13, the use of taping facilitates the
drainage process due to the increase in space between the skin and muscle
tissue, promoting the opening of lymphatic vessels and the sliding of the skin
on the fascia, mechanism that consequently contribute to improvements in
lymphatic and venous circulation.
The application of taping in its various forms aims to promote redirection of the
lymphatic circulation, reducing edema in places where it is installed16. This finding corroborates the
indications of Kase et al.17, who
reported removal of edema from the directioning of exudates toward the lymphatic
ducts and increasing the local circulation.
Furthermore, pain was not reported in the operated region in the EG. In this
case, according to the findings of Chi et al.10, postoperative treatment should be started as early as possible
to avoid possible postoperative complications such as seroma, prolonged edema,
refractory ecchymosis, and intense pain.
Some authors have already reported several physiological effects after the use of
taping such as reduction of pain or abnormal sensation and removal of lymphatic
congestion, fluid, or bleeding under the skin18-22 as supported
by our results.
There was a low occurrence of severe edema and ecchymoses in the EG. The use of a
compression plaque for the operated region, involving the entire abdominal
circumference of the patients, was associated to the application of the
lymphatic taping technique to assist in the absorption of edema with the patient
still in the surgical (transoperative) block and responsible for this low rate
of edema and ecchymoses.
Another important factor in this study was the choice of cutting the lymphatic
taping into the “fan” or “octopus” shapes. In this technique, the elastic band
is cut into 4 or 5 strips with a fixed basis of approximately 5 cm. The articles
identified in the literature using taping for edema20,21,23,24 used only
the “fan” or «octopus» cut as described here.
According to Sijmonsma25, below the base
of the “fan” (octopus) there is the possibility of triggering a skin irritation
that does not occur below loose strips. In the present study, we observed no
cases of skin irritation. Also, in accordance with the results of this study,
Martins et al.26 described no cases of
skin lesions, thus justifying the use of the fan cut for the treatment of
edema.
Another aspect to highlight is the fact that use of the taping is associated with
skin lesions or allergic reactions20;
thus, we must prudently apply taping in smaller areas in an attempt to minimize
the occurrence of these problems27.
Therefore, the “fan” or “octopus” cut is suggested during trans- and
postoperative plastic surgery care.
The treatment was initiated on the 4th postoperative day in both
groups. The choice of the initiation of treatment was based on studies of
Psillakis et al.28, who reported that the
lymphatic anastomoses are more intense between the 4th and
7th postoperative days, in addition to the studies of Mendez et
al.29, who mentioned that the
absorption of interstitial fluid is hampered until the 5th
postintervention day and returns to normal on the 10th day.
Flap adhesion occurs through a thin mesh of fibrin, which is infiltrated by
fibroblasts that transform the tenuous adhesion into a definitive adhesion by
fibrous tissue. An adhesion that allows the manipulation of the area repeatedly
only occurs on the 4th postoperative day in agreement with the
present study7.
The degree of fibrosis was lower in the EG (p = 0.0002) than in
the CG. The excess edema favors fibrosis formation. The rapid absorption of
local edema, through the action of the lymphatic taping associated with the
uniform compression of the operated region, decreased even more when the
accumulation of fluid inside the tunnels was triggered by the liposuction
cannula. With these small spaces, scar tissue formation will be less likely, as
will fibrosis formation30.
Fibrosis formation is mediated by the interaction between fibrinogenic growth
factors and pro-fibrotic cytokines in addition to other influences such as
mechanical stress, chronic inflammation, and oxidative stress31. For the EG, the use of oral and topical
antiglycans and nutritional guidelines was indicated to better control
inflammation and oxidative stress.
According to Rocha & Paula32, it is
possible to prevent or alleviate complications such as fibrosis using functional
foods. Modulating the inflammatory process, a diet rich in anti-inflammatory
agents can prevent, combat, and even reverse some of the damage caused by
inflammation36,33.
The analysis of fibrosis degree was also evaluated by contact thermography from
the 4th postoperative day, and the findings were predominantly normal
in the EG (p = 0.0002). Chi et al.10 used contact thermography as an instrument for the early
detection and classification of fibrosis.
The difference between the placements of the base of the taping in comparison to
the two regions, axillary lymph nodes and coccygeal lymph node, was analyzed in
two patients in the EG, but no visual difference was noted between the two
positions, so either was considered acceptable.
In all patients of the EG, in areas in which taping was not applied, no areas
with ecchymosis and edema were observed.
Taping should cover the entire area with edema. When applied to the skin, it
provides a greater opening of initial lymphatics, favoring the absorption of
interstitial fluid into the lymphatic channel34-36.
The low rate of fibrosis formation in the EG was due to several factors. Among
them, a preoperative conduct consisting of an anti-inflammatory diet and
restriction of foods with a high glycemic index associated with the oral use of
nutricosmetics and topical use of active antiglycants and anti-inflammatory
agents; and conduct during surgery included the use of a compression plate and
the placement of the lymphatic taping during surgery.
Individuals in the EG reacted differently in the postoperative period compared to
those in the CG, with evidence of the influence of different classifications on
the results of the statistical tests. Thus, the treatment result depends
statistically on the pre- (nutritional guidelines, use of topical and oral
nutricosmetics) and trans-operative (taping and containment foam) measures.
Our results suggest that the preoperative use of antiglycant cosmetics,
nutricosmetics, and anti-inflammatory medications associated with the
transoperative placement of lymphatic taping below the containment foam reduces
edema, ecchymosis formation, and fibrosis formation in the postoperative period.
It also decreases the number of required physiotherapy sessions and accelerates
the patient’s recovery from abdominal surgeries.
COLLABORATIONS
|
AC
|
Analysis and/or interpretation of data; final approval of the
manuscript; conception and design of the study; completion of
surgeries and/or experiments; writing the manuscript or critical
review of its contents.
|
|
AL
|
Final approval of the manuscript; writing the manuscript or critical
review of its contents.
|
|
MVTNG
|
Final approval of the manuscript.
|
|
CBS
|
Statistical analyses.
|
REFERENCES
1. International Society of Aesthetic Plastic Surgery (ISAPS)
[Internet]. 2017 [cited 2017 Ago 9]. Available from: https://www.isaps.org
2. Soncini JA, Baroudi R. Revisão da técnica de abdominoplastia com
dissecção reduzida e fixação com pontos de Baroudi. Rev Bras Cir Plást.
2016;31(2):166-71.
3. Souza LS, Harada MN, Bolognani EMC. Comparação da ocorrência de
seroma entre as técnicas de abdominoplastia convencional e em âncora nos
pacientes pós-bariátricos. Rev Bras Cir Plást.
2017;32(1):78-86.
4. Carloni R, Naudet F, Chaput B, de Runz A, Herlin C, Girard P, et al.
Are There Factors Predictive of Postoperative Complications in Circumferential
Contouring of the Lower Trunk? A Meta-Analysis. Aesthet Surg J.
2016;36(10):1143-54. DOI: http://dx.doi.org/10.1093/asj/sjw117
5. Masson IF, de Oliveira BD, Machado AF, Farcic TS, Júnior IE, Baldan
CS. Manual lymphatic drainage and therapeutic ultrasound in liposuction and
lipoabdominoplasty post-operative period. Indian J Plast Surg. 2014;47(1):70-6.
DOI: http://dx.doi.org/10.4103/0970-0358.129627
6. Macedo ACB, Oliveira SM. A atuação da fisioterapia no pré e
pós-operatório de cirurgia plástica corporal: uma revisão de literatura. Cad Esc
Saúde. 2011;1(5):169-89.
7. Lange A. Fisioterapia Dermato Funcional Aplicado à Cirurgia
Plástica. Curitiba; 2017.
8. Schwartz SI. Princípios de Cirurgia. Rio de Janeiro: Guanabara
Koogan; 1987.
9. Lange A, Chi A. Fibrose: da prevenção ao tratamento. Curitiba;
2018.
10. Chi A, Oliveira AVM, Ruh AC, Schleder JC. O uso do linfotaping,
terapia combinada e drenagem linfática manual sobre a fibrose no pós-operatório
de cirurgia plástica de abdome. Fisioter Bras.
2016;17(3):197-203.
11. Zanchet MA, Del Vecchio FB. Efeitos da bandagem kinesio taping(tm)
na recuperação de hematoma. Decorrente de distensão durante a prática do tênis:
um estudo de caso. In: Anais do XVII Congresso Brasileiro de Ciências do Esporte
e IV Congresso Internacional de Ciências dos Esporte. Porto Alegre: CONBRACE;
2011.
12. Chou YH, Li SH, Liao SF, Tang HW. Case report: Manual lymphatic
drainage and kinesio taping in the secondary malignant breast cancer-related
lymphedema in an arm with arteriovenous (A-V) fistula for hemodialysis. Am J
Hosp Palliat Care. 2013;30(5):503-6. DOI: http://dx.doi.org/10.1177/1049909112457010
13. Bosman J, Piller N. Lymph taping and seroma formation post breast
cancer. J Lymphoed. 2010;5(2):12-21.
14. Van Zuilen M. Técnicas de aplicação de bandas neuro-musculares.
Cascais: Aneid, Produtos Farmacêuticos; 2011.
15. Neves AC, Cruz AC, Pinho AM, Barreira AS, Tenreiro A, Fernandes D,,
et al. Bandas neuromusculares: um complemento na abordagem da fisioterapia
mastectomia [Internet]. Notícias de Bandas Neuromusculares; 2010 [acesso 2018
Maio 3]. Disponível em: https://www.aneid.pt/wp-content/uploads/2018/03/news_bandas_set10.pdf
16. Pyszora A, Krajnik M. Is Kinesio Taping useful for advanced cancer
lymphoedema treatment? A case report. Adv Palliat Med.
2010;9(4):141-4.
17. Kase K, Lemos TV, Dias EM. Kinesio Taping - Introdução ao Método e
Aplicações Musculares. São Paulo: Andreoli; 2013.
18. Tsai HJ, Hung HC, Yang JL, Huang CS, Tsauo JY. Could Kinesio tape
replace the bandage in decongestive lymphatic therapy for breast-cancer-related
lymphedema? A pilot study. Support Care Cancer. 2009;17(11):1353-60. DOI:
http://dx.doi.org/10.1007/s00520-009-0592-8
19. Aguilar-Ferrándiz ME, Castro-Sánchez AM, Matarán-Peñarrocha GA,
Guisado-Barrilao R, García-Ríos MC, Moreno-Lorenzo C. A randomized controlled
trial of a mixed Kinesio taping-compression technique on venous symptoms, pain,
peripheral venous flow, clinical severity and overall health status in
postmenopausal women with chronic venous insufficiency. Clin Rehabil.
2014;28(1):69-81. DOI: http://dx.doi.org/10.1177/0269215512469120
20. Smykla A, Walewicz K, Trybulski R, Halski T, Kucharzewski M, Kucio
C, et al. Effect of Kinesiology Taping on breast cancer-related lymphedema: a
randomized single-blind controlled pilot study. Biomed Res Int.
2013;2013:767106. DOI: http://dx.doi.org/10.1155/2013/767106
21. Pekyavas NÖ, Tunay VB, Akbayrak T, Kaya S, Karatas M. Complex
decongestive therapy and taping for patients with postmastectomy lymphedema: a
randomized controlled study. Eur J Oncol Nurs. 2014;18(6):585-90. DOI: http://dx.doi.org/10.1016/j.ejon.2014.06.010
22. Tozzi U, Santagata M, Sellitto A, Tartaro GP. Influence of
Kinesiologic Tape on Post-operative Swelling After Orthognathic Surgery. J
Maxillofac Oral Surg. 2016;15(1):52-8. DOI: http://dx.doi.org/10.1007/s12663-015-0787-0
23. Taradaj J, Halski T, Rosinczuk J, Dymarek R, Laurowski A, Smykla A.
The influence of Kinesiology Taping on the volume of lymphoedema and manual
dexterity of the upper limb in women after breast cancer treatment. Eur J Cancer
Care (Engl). 2016;25(4):647-60. DOI: http://dx.doi.org/10.1111/ecc.12331
24. Gatt M, Willis S, Leuschner S. A meta-analysis of the effectiveness
and safety of kinesiology taping in the management of cancer-related
lymphoedema. Eur J Cancer Care (Engl). 2017;26(5). DOI: http://dx.doi.org/10.1111/ecc.12510
25. Sijmonsma J. Lymph Taping. In: Sijmonsma J, ed. Lymph taping. Hof
van Twente: Fysionair; 2010. p. 57-84.
26. Martins Jde C, Aguiar SS, Fabro EA, Costa RM, Lemos TV, de Sá VG, et
al. Safety and tolerability of Kinesio Taping in patients with arm lymphedema:
medical device clinical study. Support Care Cancer. 2016;24(3):1119-24. DOI:
http://dx.doi.org/10.1007/s00520-015-2874-7
27. Pivetta HMF, Petter GN, Penna GB, Martins TNO, Santos LF, Pautz ACG.
Efeitos do Kinesio Taping sobre o edema linfático. Fisioter Bras.
2017;3(18):382-90.
28. Psillakis JM, de Jorge FB, Villardo R, de Albano AM, Martins M,
Spina V. Water and electrolyte changes in autogenous skin grafts. Discussion of
the so-called "plasmatic circulation". Plast Reconstr Surg. 1969;43(5):500-3.
PMID: 4889412 DOI: http://dx.doi.org/10.1097/00006534-196905000-00008
29. Mendez U, Brown EM, Ongstad EL, Slis JR, Goldman J. Functional
recovery of fluid drainage precedes lymphangiogenesis in acute murine foreleg
lymphedema. Am J Physiol Heart Circ Physiol. 2012;302(11):H2250-6. PMID:
22427513 DOI: http://dx.doi.org/10.1152/ajpheart.01159.2011
30. Gantwerker EA, Hom DB. Skin: histology and physiology of wound
healing. Facial Plast Surg Clin North Am. 2011;19(3):441-53. DOI: http://dx.doi.org/10.1016/j.fsc.2011.06.009
31. Mu X, Bellayr I, Walters T, Li Y. Mediators leading to fibrosis -
how to measure and control them in tissue engineering. Oper Tech Orthop.
2010;20(2):110-8. DOI: http://dx.doi.org/10.1053/j.oto.2009.10.003
32. Rocha CL, Paula VB. Nutrição funcional no pós-operatório de cirurgia
plástica: enfoque na prevenção de seroma e fibrose. Rev Bras Cir Plást.
2014;29(4):609-24.
33. Pujol AP. Nutrição aplicada à estética. Rio de Janeiro: Rubio;
2011.
34. Fernandes J, Chandía PY, Garcia FE. Tape Neuro Muscular -
Aplicaciones Prácticas. Buenos Aires: TNM Argentina; 2016.
35. Bosman J. Lymph taping for lymphoedema: an overview of the treatment
and its uses. Br J Community Nurs. 2014;Suppl:S12, S14, S16-8. DOI: http://dx.doi.org/10.12968/bjcn.2014.19.Sup4.S12
36. Pamuk U, Yucesoy CA. MRI analyses show that kinesio taping affects
much more than just the targeted superficial tissues and causes heterogeneous
deformations within the whole limb. J Biomech. 2015;48(16):4262-70. DOI:
http://dx.doi.org/10.1016/j.jbiomech.2015.10.036
1. Instituto Universitário Italiano de Rosário,
Santa Fe, Argentina.
2. Pontifícia Universidade Católica do Paraná,
Curitiba, PR, Brazil.
3. Universidade Tuiuti do Paraná, Curitiba, PR,
Brazil.
4. Instituto Marcus Thomé, Ponta Grossa, PR,
Brazil.
5. Faculdade IBRATE, Curitiba, PR,
Brazil.
6. Centro Universitário de Volta Redonda, Rio de
Janeiro, RJ, Brazil.
7. Associação Médica Brasileira, São Paulo, SP,
Brazil.
8. Sociedade Brasileira de Cirurgia Plástica, São
Paulo, SP, Brazil.
9. Universidade Estadual de Ponta Grossa, Ponta
Grossa, PR, Brazil.
10. Universidade Tecnológica Federal do Paraná,
Curitiba, PR, Brazil.
Corresponding author, Anny Chi, Rua
Nestor Guimarães, 77 - Ponta Grossa, PR, Brazil. Zip Code 84040-130. E-mail:
annychi10@hotmail.com
Article received: October 14, 2017.
Article accepted: September 5, 2018.
Conflicts of interest: none.