INTRODUCTION
The scalp is an area of considerable thickness, little elasticity, and abundant
vascularization. These characteristics make surgical procedures in the area
difficult due to the appearance of complications such as profuse intraoperative
bleeding. We present a case of NF1 in which a wide scalp resection was performed
due to the presence of multiple neurofibromas and reconstruction with advanced
flaps plus partial skin autograft (PSA).
CASE HISTORY
A 44-year-old male patient, with a history of NF1, hypertension, and type 2
diabetes mellitus, came to the Department of Plastic and Reconstructive Surgery
due to the presence of multiple tumors of varying shapes and sizes on the scalp,
some of them with purulent discharge and pain. Ten years ago, local surgical
resection of neurofibromas in the nose was performed. On physical examination,
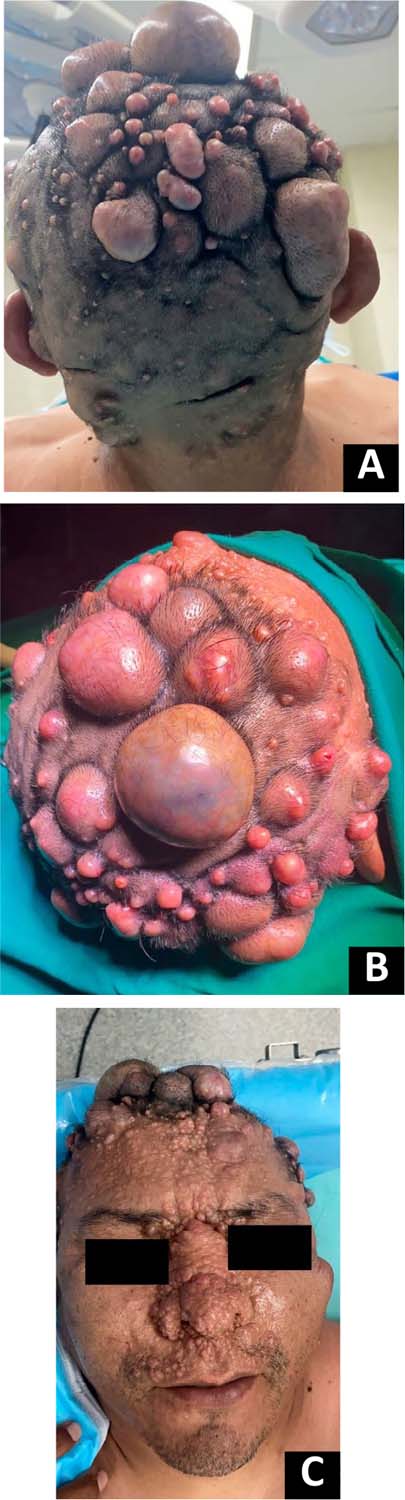
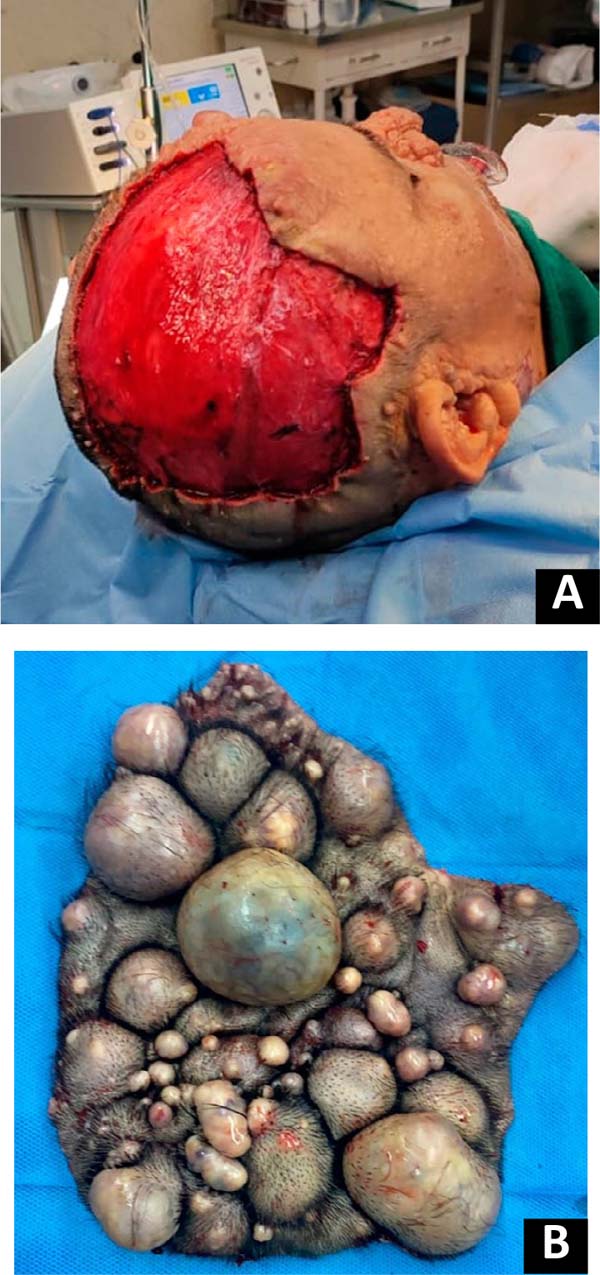
multiple neurofibromas were seen on the scalp and face (Figure 1), café-au-lait spots on the axillary flexure, and
Lisch nodules.
Figura 1 - A: Neurofibromas elevados, pedunculados e de base
larga, com até aproximadamente 5cm de diâmetro e sensíveis à
palpação, são observados no couro cabeludo. B: Vista
superior dos neurofibromas no couro cabeludo. C:
Múltiplos neurofibromas de até aproximadamente 1cm de diâmetro podem
ser observados na metade superior da face e nariz.
Figura 1 - A: Neurofibromas elevados, pedunculados e de base
larga, com até aproximadamente 5cm de diâmetro e sensíveis à
palpação, são observados no couro cabeludo. B: Vista
superior dos neurofibromas no couro cabeludo. C:
Múltiplos neurofibromas de até aproximadamente 1cm de diâmetro podem
ser observados na metade superior da face e nariz.
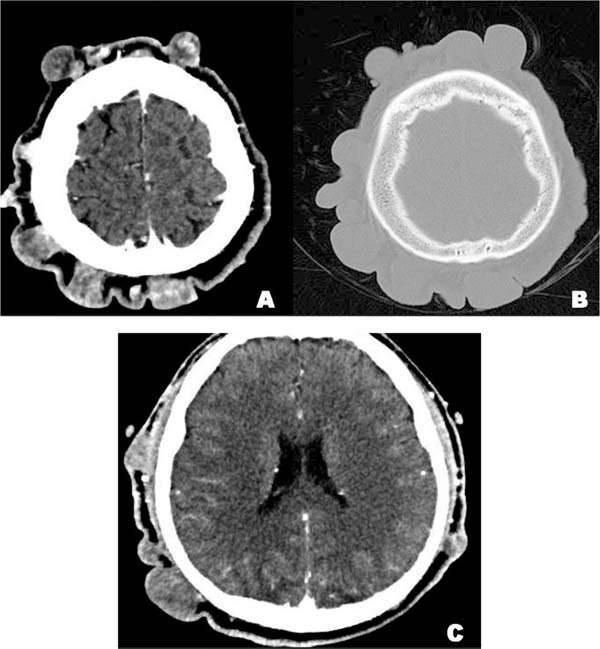
In the Computerized Tomography (CT) of the head and neck, scattered nodular
formations of different sizes with a density equivalent to soft tissue are
observed in the scalp, and with irregular enhancement in the image after
contrast administration, characteristics corresponding to neurofibromas (Figure 2A). The surrounding bone tissue was
intact, without the presence of erosions or signs of intracranial extension
(Figure 2B/2C).
Figura 2 - Tomografia computadorizada de cabeça e pescoço. A) Neurofibromas
múltiplos no couro cabeludo, com tamanhos de 3 x 4mm a 48 x 25mm. B)
Na janela óssea, não são observadas erosões ou infiltrações do
tecido ósseo circundante. C) Nenhuma evidência de extensão
intracraniana.
Figura 2 - Tomografia computadorizada de cabeça e pescoço. A) Neurofibromas
múltiplos no couro cabeludo, com tamanhos de 3 x 4mm a 48 x 25mm. B)
Na janela óssea, não são observadas erosões ou infiltrações do
tecido ósseo circundante. C) Nenhuma evidência de extensão
intracraniana.
Due to the clinic, the size, and wide distribution of the neurofibromas, it was
decided to perform a partial surgical excision of the scalp. The procedure
involved a two-stage approach due to the impracticality of individually excising
extensive and progressive neurofibromas on the scalp. The first stage included
the radical resection of affected scalp areas, followed by the placement of a
PSA for rapid coverage.
This decision considered patient preferences and the explanation of treatment
options. Free tissue transfer was chosen over local flaps to address scalp
defects, aiming to prevent adherence failure and defect enlargement. The use
of
a PSA for scalp reconstruction was preferred for its simplicity and
functionality.
Regarding the surgical intervention. After administration of general anesthesia
with endotracheal intubation, the lesions were marked with methylene blue, then
Klein’s tumescent solution was infiltrated in the subperiosteal plane. With a
number 15 scalpel, a cut is made perpendicular to the scalp on the pre-marked
design. With electrocautery, dissection was performed up to the periosteal
plane, removing the scalp with the neurofibromas (Figure 3). Advancement flaps are made with Baroudi sutures
(progressive tension sutures) on all edges, achieving the reduction of the
resulting defect.
Figura 3 - Área do couro cabeludo extraída até o periósteo.
Figura 3 - Área do couro cabeludo extraída até o periósteo.
The PSA was harvested with an electrical dermatome, the donor area was the
anterolateral aspect of the right thigh. The PSA was prepared by placing it on
paraffin gauze, with diffuse micro-openings made with a scalpel 11, and after
placement, it was sutured with a tie-over dressing. A hydrocolloid dressing and
a compressive bandage were placed on the donor area. The patient had
postoperative recovery without complications.
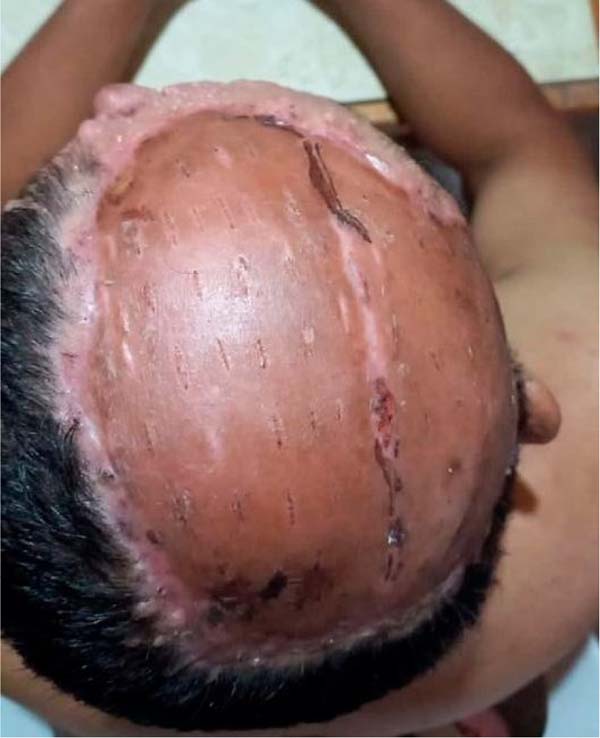
The PSA is discovered on day 10, finding good integration, without the presence
of residual ulcers, hematomas, or seromas (Figure 4). In the donor area, it was found in the process of
re-epithelialization, so it was decided to place a new hydrocolloid dressing
plus an occlusive bandage. The patient was discharged, and he did not require
any type of coverage over the donor area and the PSA had a good evolution.
Figura 4 - Autoenxerto parcial de pele após 10 dias.
Figura 4 - Autoenxerto parcial de pele após 10 dias.
DISCUSSION
NF1 is an autosomal dominant genetic disease with multiorgan involvement and has
an approximate global prevalence of 1 case per 3,000 live births1. All cases have a germline
mutation of the NF1 gene, however, there is a high variability in the
presentation of clinical characteristics1. The main clinical feature of NF1 is neurofibroma, a
nerve sheath tumor formed from spinal, peripheral, or cranial nerves.
Neurofibromas are the most frequent benign tumors in NF1 and different types have
been described, such as cutaneous, subcutaneous, and plexiform
neurofibromas2. Other
clinical features include pigmentary lesions (café au lait macule), skeletal
abnormalities, behavioral abnormalities, and low-grade gliomas1.
The most common and striking dermatological finding of NF1 is the presence of
several cutaneous neurofibromas (cNFs) of different sizes and shapes2. cNFs have several cellular
components (Schwann cells, fibroblasts, macrophages, and mast cells) and are
supported by an extracellular matrix within the dermis1,3.
cNFs have several stages depending on their clinical appearance: the nascent
stage (not clinically observable), the flat stage (thinning or hyperpigmentation
of the skin), the sessile stage (raised papule), the globular stage (a base of
20 to 30 mm and height), and the pedunculate stage (dermal contents supported
by
a visible stalk)4. Common
neurological concomitant symptoms of cNFs are pain and itching, and inadequate
drying after wetting can cause maceration, skin breakdown, and superficial
infections5.
It should be noted that physical disfigurement from multiple neurofibromas is
associated with less quality of life due to psychosocial burden, low
self-esteem, and interference with daily, social, and economic
activities6. This is
why treatment is justified, especially with the presence of severe symptoms
and/or aesthetic disfigurement4. In our case, the presence of multiple neurofibromas was
evidenced, mainly in the scalp, which caused an important aesthetic
disfigurement in the head, concomitant pain, and signs of infection such as
purulent discharge.
Currently, there is no accepted gold standard for the treatment of cNFs5. For the choice of treatment,
the type, size, number, and location of the neurofibroma should be
considered4. Surgical
excision is still the most effective method, however, it does not prevent the
formation of future neurofibromas5. People with NF1 generally have an extensive tumor burden
that covers a large area of their body surface and due to the progression of
the
disease, the surgical procedures that are performed are usually
multi-stage4.
In our case, due to the progression, and extensive extension of the neurofibromas
in the scalp, individual excision of each neurofibroma was not feasible, so it
was decided to perform a more robust surgical approach in 2 stages, first with
radical resection of the neurofibroma. Affected scalp area and subsequent
placement of the PSA, obtaining favorable results in the short term7. This treatment decision was
made taking into account the patient’s preferences and needs, who chose after
having all of his treatment options explained to him.
Another therapeutic technique that could have been used is local scalp flaps
(advancement or rotation) previously expanded with tissue expanders since they
present certain advantages such as a better aesthetic finish in color and
texture, resistance, and long-term durability9. However, to cover scalp defects it is advisable to
perform free tissue transfer instead of local flaps, since the failure of
adherence to the flaps can increase the defect10. For the reconstruction of the scalp a PSA was
placed as it was the simplest and most functional form that ensured a rapid
coverage of the exposed area10. However, identification and excision of these tumors early in
their development would have given better aesthetic results.
Although the use of PSA is a convenient technique for the reconstruction of large
scalp defects, these grafts have some disadvantages11. Alopecia would be one of the future
consequences of the placement of the PSA12. An autologous hair transplant from preserved areas of
the scalp could be an alternative for alopecia, however, this technique is
reserved for small areas13. In
addition, Schwann cells are considered to be the tumor cells of cNFs,2 and they make up an important
cell population in the dermis14, therefore, their presence in the PSA and autologous hair
transplant could lead to the future development of cNFs in the area.
Furthermore, PSA mobilization is limited and not similar to that of the normal
scalp, and they are sometimes quite fragile and susceptible to shear
forces.12 In our
patient we prioritized a reconstruction that would allow us to cover the entire
scalp and easily observe the appearance of tumors in the future.
CONCLUSION
In this report, we describe the surgical management of multiple cNFs on the scalp
in a patient with NF1. The resection of the affected scalp and the
reconstruction of the resected area with advancement flaps and placement of
partial skin autograft gave acceptable results that will have a positive impact
on the patient’s quality of life in the future.
REFERENCES
1. Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson
KJ. Neurofibromatosis type 1. Nat Rev Dis Primers.
2017;3:17004.
2. Allaway RJ, Gosline SJC, La Rosa S, Knight P, Bakker A, Guinney J,
et al. Cutaneous neurofibromas in the genomics era: current understanding and
open questions. Br J Cancer. 2018;118(12):1539-48.
3. LE LQ, Kesterson RA, Gutmann DH. Defining the Research Landscape for
Dermal Neurofibromas. Oncol Times. 2016;38(18):14-5.
4. Chamseddin BH, Hernandez LN, Solorzano D, Vega J, Le LQ. Robust
surgical approach for cutaneous neurofibroma in neurofibromatosis type 1. JCI
Insight. 2019;4(11):e128881.
5. Chamseddin BH, Le LQ. Management of cutaneous neurofibroma: current
therapy and future directions. Neurooncol Adv. 2019;2(Suppl
1):i107-i116.
6. Taylor LA, Lewis VL Jr. Neurofibromatosis Type 1: Review of
Cutaneous and Subcutaneous Tumor Treatment on Quality of Life. Plast Reconstr
Surg Glob Open. 2019;7(1):e1982.
7. Park H, Min J, Oh TS, Jeong WS, Choi JW. Scalp Reconstruction
Strategy Based on the Etiology of the Scalp Defects. J Craniofac Surg.
2022;33(8):2450-4.
8. Costa DJ, Walen S, Varvares M, Walker R. Scalp Rotation Flap for
Reconstruction of Complex Soft Tissue Defects. J Neurol Surg B Skull Base.
2016;77(1):32-7.
9. Bas S, Oner C, Eren HI, Hacikerim Karsidag S, Yilmaz A.
Reconstruction of Complex Scalp Defects in Different Locations: Suggestions for
Puzzle. Sisli Etfal Hastan Tip Bul. 2021;55(3):349-58.
10. Desai SC, Sand JP, Sharon JD, Branham G, Nussenbaum B. Scalp
reconstruction: an algorithmic approach and systematic review. JAMA Facial Plast
Surg. 2015;17(1):56-66.
11. Lee KK, Mehrany K, Swanson NA. Scalp Reconstruction. In: Rohrer TE,
Cook JL, Kaufmann A, eds. Flaps and Grafts in Dermatologic Surgery. Chapter 10.
Edinburgh: Saunders; 2007. p. 117-24.
12. Lane C, Lin A, Goyal N. Scalp and Calvarium Reconstruction.
Otolaryngol Clin North Am. 2023;56(4):741-55.
13. Leedy JE, Janis JE, Rohrich RJ. Reconstruction of acquired scalp
defects: an algorithmic approach. Plast Reconstr Surg.
2005;116(4):54e-72e.
14. Bray ER, Chéret J, Yosipovitch G, Paus R. Schwann cells as
underestimated, major players in human skin physiology and pathology. Schwann
cells as underestimated, major players in human skin physiology and pathology.
Exp Dermatol. 2020;29(1):93-101.
1. Universidad Nacional De San Agustín de
Arequipa, Peru
Corresponding author: Elizbet Susan
Montes-Madariaga Urb. José Luis Bustamante y Rivero, Cerro Colorado,
Arequipa, Peru, E-mail: emontesma@unsa.edu.pe