INTRODUCTION
Coronavirus disease 2019 (COVID-19) emerged in China and spread globally with
sustained human-to-human transmission1.
Due to its highly contagious nature, unprecedented global spread, aggressive
clinical presentation, and the lack of effective treatment, the acute
coronavirus 2 (SARS-CoV-2) respiratory infection syndrome is causing the loss
of
thousands of lives and repercussions unmatched in health systems worldwide2.
The infection caused by COVID-19 is a highly transmissible disease that presents
a significant risk for both patients and health professionals3. Recently, it was demonstrated that high
levels of the virus are present in respiratory secretions during the
pre-symptomatic period, which can last from days to weeks before the
characteristic symptoms of COVID-194. The
virus’s ability to be transmitted by people without symptoms is one of the main
reasons for the pandemic5.
The diagnosis of COVID-19 is made using clinical, laboratory, and radiological
characteristics6. As the signs and
radiological findings of COVID-19 are nonspecific, infection with SARS-CoV-2
must be confirmed by laboratory tests. Polymerase chain reaction tests with
reverse transcriptase (RT-PCR) are the gold standard for the diagnosis of
COVID-19. However, it is challenging to collect tests, and its results are not
immediately available7. The rapid
diagnostic tests for COVID-19 IgM / IgG were developed using lateral flow
technology to find antigens from the SARS-CoV-2 virus and detect antibodies
produced by patients infected with COVID-198.
Screening tests are widely used to assess the probability of members of a defined
population having a specific disease; with few exceptions, screening tests do
not diagnose the disease9. The rapid
serological diagnostic test performed at the time of admission, before elective
surgery, is among the most widely used pre-operative screening methods for
COVID-1910-18.
OBJECTIVES
The aim of this study is to evaluate the role of rapid tests for COVID-19
antibodies in outpatients being admitted for aesthetic plastic surgery.
METHODS
A systematic review of the literature was performed using the search engines in
PubMed, Web of Science, and SciELO journals, for studies with animals and humans
published from December 2019 to July 30, 2020. We consider specific terms about
COVID-19 or SARS- CoV-2 and plastic surgery. The following descriptors were
used: “plastic surgery”, “elective surgery”, “COVID-19”, “COVID-19 diagnostic
test”, “COVID-19 blood antibody test”, “SARS-CoV-2 test”. Many terms and words
were displayed similarly when searching for articles. Words like
“pre-operative,” “surgical,” and “surgery” showed similar results. The results
of the words and phrases investigated were analyzed by quantity and quality.
Documents written in English, Spanish, French, Italian, and Portuguese were
included. Videos, posters, and letters to the editor were disregarded. Two
researchers independently selected the relevant articles through the evaluation
of titles and abstracts. The third researcher reviewed relevant articles. Data
on the level of evidence, sensitivity, specificity, and predictive values of
rapid diagnostic tests were collected.
This study follows Helsinki’s declaration and does not need to be evaluated by an
ethics committee since it does not directly involve collecting data or tissues
from human beings, only research conducted exclusively with scientific
texts.
RESULTS
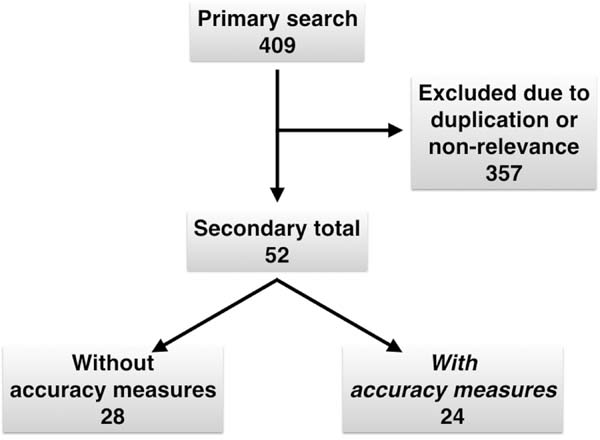
Using our active search strategy, the database review found 409 articles (Figure 1). A total of 357 studies were
duplicated or considered not relevant to our research question. Among the
remaining articles, 28 were studies without information on the accuracy of rapid
diagnostic tests, and 24 were studies describing measures of accuracy7,8,19-40.
Figure 1 - Algorithm of a systematic review.
Figure 1 - Algorithm of a systematic review.
The level of evidence varied from V to III. The sensitivity varied from 18.4 to
100%, the specificity varied from 94 to 100%, the positive predictive value
varied between 19.7 and 100%, and the negative predictive value was between 20
and 100%.
DISCUSSION
The limited experience accumulated during the COVID-19 pandemic has shown that
the management of all medical conditions, including elective surgeries, has
undergone some degree of change2. We all
want to go back to work without the COVID-19 spectrum. During the extraordinary
conditions of the COVID-19 pandemic, the ideal strategies for treating aesthetic
patients individually are unknown. There is no consensus in the literature
regarding pre-operative care, except that all patients should be screened for
symptoms before being presented to the operating room, and those who report
symptoms of COVID-19 should be referred for further evaluation.
The rapid diagnostic test can be produced quickly and cheaply. This qualitative
test is small and portable, usually similar to a pregnancy test, showing to the
user colored lines to indicate positive or negative results8. Rapid diagnostic tests do not measure the number of
antibodies in the patient’s serum or whether these antibodies can protect
against future infections. However, they do have the ability to detect exposure
and can identify asymptomatic people and people who have cleared the virus. Many
of the rapid diagnostic tests available so far lack analytical performance
concerning sensitivity and specificity and need to be better validated before
being used preoperatively21.
For a medical diagnosis, the test’s sensitivity is its ability to correctly
identify those with the disease (true positive rate), while the test’s
specificity is its ability to accurately identify those without the disease
(true negative rate). In this research, sensitivity ranged from 18.4 to 100%,
reflecting a potential inability to identify people who have antibodies to
COVID-19 correctly. Specificity varied between 94 and 100%, demonstrating a high
ability to identify all patients who do not have COVID-19 antibodies.
The negative predictive value is the probability that patients with a negative
result in a rapid diagnostic test do not have COVID-19 antibodies; in our
research, their values were between 20 and 100%, we can say that, in some
circumstances, 80% of individuals with a negative test may have COVID-19
antibodies. Positive predictive value is the likelihood that individuals with
a
rapid positive diagnostic screening test will have the disease; in our research,
its variation was between 60 and 100%. Consequently, we can affirm that, in some
circumstances, 40% of the individuals with positive rapid tests may not have
antibodies to COVID- 19. Therefore, the rapid test results seem to be
scientifically unreliable, and the recommendation to perform this testing in
a
generalized way by patients or hospital institutions seems inadequate.
It is estimated that SARS-CoV-2 IgM antibodies can be detected in a blood sample
after three days and IgG antibodies eight days after the onset of symptoms7. The seroconversion rate for IgM and IgG
was described as 82.7% and 64.7%, respectively5. To date, we do not know whether everyone who has recovered from
COVID-19 has developed antibodies, and we do not know to what extent these
antibodies protect patients from reinfection. The antibody tests do not detect
an active infection but look for signs that a person has been previously
infected, as shown by the antibodies his immune system has produced to fight
the
coronavirus. With other diseases, the presence of antibodies usually means
acquired immunity for at least some period, but this is not yet known in the
case of COVID-194.
Patients should be screened only if a positive test results in mandatory action.
This is not the case for rapid diagnostic tests for COVID-19 before cosmetic
surgery because the procedure will be performed regardless of antibody detection
status. In the case of the new COVID-19 virus and the SARS-CoV-2 disease it
causes in humans, the objective of pre-operative testing would be
straightforward: to identify infected patients and isolate them by postponing
their surgeries, trying to reduce the morbidities of the procedure and thus
reducing the risk of infection for health professionals8. Nevertheless, without a perfect test, false positives and
false negatives can lead to significantly worse outcomes.
Both false positives and false negatives pose their own unique dangers wherever
testing occurs, but false negatives are particularly dangerous for COVID-19.
Two
weeks after surgery, some patients may be positive for COVID-19; despite the
negative pre-operative result, important medico-legal implications may arise.
Was the infection contracted during hospitalization? Did the surgeon or his team
contaminate the patient during outpatient postoperative follow-up? Did
seroconversion occur because of the surgery-induced immunosuppression? A
negative result in a rapid diagnostic test for COVID-19 performed preoperatively
can be dangerous medico-legal evidence for surgeons and hospital entities.
Antibody tests are versatile: these serological tests are of critical importance
to determine seroprevalence, prior exposure and to identify highly reactive
human donors for the generation of therapeutic convalescent serum4. They will also support contact screening
and screening for healthcare professionals to identify those who are already
immune.
It is plausible that several limitations may have influenced the results obtained
in this research. The exclusion of articles in Asian languages is one of them
since much of the knowledge about COVID-19 comes from this geographic area.
However, there was none among the researchers with knowledge of these languages
, and we consider that electronic translators are not reliable. However, many
of these studies would provide information with limited external validity for
patients in the Americas since COVID-19 mutations are frequent, and most of the
rapid diagnostic tests used there are not available on our continent. A
well-designed systematic review benefits the evolution of knowledge, identifying
a lack of scientific information and providing a synopsis of the available
evidence. The credibility of systematic reviews can be compromised by reporting
bias, which arises when the results’ nature influences the dissemination of
published articles. Our findings are based on a limited number of articles;
therefore, the results of such analysis should be treated with utmost
caution.
Controlled clinical trials are lacking, and future studies should examine the
safety and efficacy of rapid diagnostic tests for COVID-19 to obtain more
consistent results and establish recommendations for their appropriate use.
CONCLUSION
The COVID-19 IgM / IgG rapid diagnostic tests appear to be inaccurate. We found
no evidence to support COVID-19 or SARS-CoV-2 antibodies’ rapid testing to
screen outpatients for cosmetic plastic surgery. Future studies on the subject
are needed to validate different laboratory diagnostic tests.
REFERENCES
1. Contini C, Di Nuzzo M, Barp N, Bonazza A, De Giorgio R, Tognon M, et
al. The novel zoonotic COVID-19 pandemic: an expected global health concern.
J
Infect Dev Ctries. 2020 Mar;14(3):254-64. DOI:
https://doi.org/10.3855/jidc.12671
2. Patel V, Jimenez E, Cornwell L, Tran T, Paniagua D, Denktas AE, et
al. Cardiac surgery during the COVID-19 pandemic: perioperative considerations
and triage recommendations. J Am Heart Assoc. 2020 Jul;9(13):e017042. DOI:
https://doi.org/10.1161/jaha.120.017042
3. He W, Yi GY, Zhu Y. Estimation of the basic reproduction number,
average incubation time, asymptomatic infection rate, and case fatality rate
for
COVID-19: meta-analysis and sensitivity analysis. J Med Virol. 2020
Mai;92(1):2543-50. DOI: https://doi.org/10.1002/jmv.26041
4. Kim SE, Jeong HS, Yu Y, Shin SU, Kim S, Oh TH, et al. Viral kinetics
of SARS-CoV-2 in asymptomatic carriers and presymptomatic patients. Int J Infect
Dis. 2020 Jun;95:441-3.
5. Wong MC, Teoh JY, Huang J, Wong SH. Strengthening early testing and
surveillance of COVID-19 to enhance identification of asymptomatic patients.
J
Infect. 2020 Ago;81(2):E112-3. DOI:
https://doi.org/10.1016/j.jinf.2020.05.048
6. Luo N, Zhang H, Zhou Y, Kong ZX, Sun WH, Huang N, et al. Utility of
chest CT in diagnosis of COVID-19 pneumonia. Diagn Interv Radiol. 2020
Mai;26:437-42. DOI: https://doi.org/10.5152/dir.2020.20144
7. Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and
clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2
infection diagnosis. J Med Virol. 2020 Fev;92(9):1518-24. DOI:
https://doi.org/10.1002/jmv.25727
8. Van Elslande J, Houben E, Depypere M, Bracknier A, Desmet S, André
E, et al. Diagnostic performance of 7 rapid IgG/IgM antibody tests and the
Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect. 2020
Ago;26(8):1082-7. DOI:
https://doi.org/10.1016/j.cmi.2020.05.023
9. Maxim LD, Daniel Maxim L, Niebo R, Utell MJ. Screening tests: a
review with examples. Inhal Toxicol. 2014 Set;26(13):811-28. DOI:
https://doi.org/10.3109/08958378.2014.955932
10. The Aesthetic Society (AS). COVID-19 updates [Internet]. Garden
Grove: AS; 2020; [acesso em 2020 Ago 01]. Disponível em: https://www.surgery.org/professionals/covid-19/covid-19-updates
11. Kaye K, Paprottka F, Escudero R, Casabona G, Montes J, Fakin R, et
al. Elective, non-urgent procedures and aesthetic surgery in the wake of
SARS-COVID-19: considerations regarding safety, feasibility and impact on
clinical management. Aesthetic Plast Surg. 2020 Mai;44:1014-42. DOI:
https://doi.org/10.1007/s00266-020-01752-9
12. Flexman AM, Abcejo AS, Avitsian R, Sloovere V, Highton D, Juul N, et
al. Neuroanesthesia practice during the COVID-19 pandemic: recommendations from
Society for Neuroscience in Anesthesiology and Critical Care (SNACC). J
Neurosurg Anesthesiol. 2020 Jul;32(3):202-9. DOI:
https://doi.org/10.1097/ANA.0000000000000691
13. Zeegen EN, Yates AJ, Jevsevar DS. After the COVID-19 pandemic:
returning to normalcy or returning to a new normal?. J Arthroplasty. 2020
Jul;35(7 Supl 1):S37-S41. DOI:
https://doi.org/10.1016/j.arth.2020.04.040
14. Liu Z, Zhang Y, Wang X, Zhang D, Diao D, Chandramohan K, et al.
Recommendations for surgery during the novel coronavirus (COVID-19) epidemic.
Indian J Surg. 2020 Abr;1-5.
15. Paraiso MFR, Brown J, Abrão MS, Dionisi H, Rosenfield RB, Lee TM, et
al. Surgical and clinical reactivation for elective procedures during the COVID
era: a global perspective. J Minim Invasive Gynecol. 2020 Jul;27(5):1188-95.
DOI: https://doi.org/10.1016/j.jmig.2020.05.012
16. Carugno J, Di Spiezio Sardo A, Alonso L, Haimovich S, Campo R, De
Angelis C, et al. COVID-19 Pandemic. Impact on hysteroscopic procedures: a
consensus statement from the global congress of hysteroscopy scientific
committee. J Minim Invasive Gynecol. 2020 Jul;27(5):988-92. DOI:
https://doi.org/10.1016/j.jmig.2020.04.023
17. Meng Y, Leng K, Shan L, Guo M, Zhou J, Tian Q, et al. A clinical
pathway for pre-operative screening of COVID-19 and its influence on clinical
outcome in patients with traumatic fractures. Int Orthop. 2020
Ago;44(8):1549-55.
18. Ren X, Chen B, Hong Y, Liu W, Jiang Q, Yang J, et al. The challenges
in colorectal cancer management during COVID- 19 epidemic. Ann Transl Med. 2020
Abr;8(7):498. DOI: https://doi.org/10.21037/atm.2020.03.158
19. Jacofsky D, Jacofsky EM, Jacofsky M. Understanding antibody testing
for COVID-19. J Arthroplasty. 2020 Jul;35(7 Supl 1):S74-S81. DOI:
https://doi.org/10.1016/j.arth.2020.04.055
20. Ragó Z, Szijjártó L, Duda E, Bella Z. Opportunity of periodic
monitoring of COVID-19 patients, asymptomatic virus carriers, and postinfectious
individuals with IgM/IgG rapid antibody tests among healthcare workers during
SARS-CoV-2 pandemic. Orv Hetil. 2020 Mai;161(21):854-60.
21. Shen B, Zheng Y, Zhang X, Zhang W, Wang D, Jin J, et al. Clinical
evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2
IgM/IgG. Am J Transl Res. 2020;12(4):1348-54.
22. Spicuzza L, Montineri A, Manuele R, Crimi C, Pistorio MP, Campisi R,
et al. Reliability and usefulness of a rapid IgM-IgG anti- body test for the
diagnosis of SARS-CoV-2 infection: a preliminary report. J Infect. 2020
Ago;81(2):53-4. DOI: https://doi.org/10.1016/j.jinf.2020.04.022
23. Cassaniti I, Novazzi F, Giardina F, et al. Performance of VivaDiag
COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute
patients referring to emergency room department. J Med Virol. 2020
Mar;92(10):1724-7. DOI: https://doi.org/10.1002/jmv.25800
24. Padoan A, Cosma C, Sciacovelli L, Faggian D, Plebani M. Analytical
performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and
antibody kinetics. Clin Chem Lab Med. 2020;58(7):1081-8. DOI:
https://doi.org/10.1515/cclm-2020-0443
25. Rosa MI, Prestes GS, Macedo ACL, Colonetti T, Uggioni MLR, Grande
AJ. Accuracy of rapid IgM and IgG antibody test for SARS-CoV-2 infection
diagnosis: a systematic review and meta analysis. Braz J Infect Dis. 2020. DOI:
https://doi.org/10.37766/inplasy2020.4.0099
26. Pulia MS, O'Brien TP, Hou PC, Schuman A, Sambursky R. Multi-tiered
screening and diagnosis strategy for COVID-19: a model for sustainable testing
capacity in response to pandemic. Ann Med. 2020
Ago;52(5):207-14.
27. Castro R, Luz PM, Wakimoto MD, Veloso VG, Grinsztejn B, Perazzo H.
COVID-19: a meta- analysis of diagnostic test accuracy of commercial assays
registered in Brazil. Braz J Infect Dis. 2020
Mar/Abr;24(2):180-7.
28. Saenz-Flor KV, Santafe LM. Concordance of "rapid" serological tests
and IgG and IgM chemiluminescence for SARS-COV-2. medRxiv [Internet]. 2020 Jun
03; [Epub preprint]. DOI: https://doi.org/10.1101/2020.06.01.20114884
29. Prazuck T, Colin M, Giachè S, Gubavu C, Seve A, Rzpecki V, et al.
Evaluation of performance of two SARS-CoV-2 rapid whole-blood finger-stick
IgM-IgG combined antibody tests. medRxiv [Internet]. 2020 May 27; [Epub
preprint]. DOI: https://doi.org/10.1101/2020.05.27.20112888
30. Di Lorenzo G, Toniolo P, Lurani C, Foresti L, Carrisi C. Evaluating
the adequacy of Prima Covid-19 IgG/IgM Rapid Test for the assessment of exposure
to SARS-CoV-2 virus. medRxiv [Internet]. 2020 Jun 03; [Epub preprint]. DOI:
https://doi.org/10.1101/2020.05.30.20117424
31. Ying, Yue-Ping L, Bo D, Fei-Fei R, Yue W, Jinya D, et al. Diagnostic
indexes of a rapid IgG/IgM combined antibody test for SARS-CoV-2. medRxiv
[Internet]. 2020 Mar 30; [Epub preprint]. DOI: https://doi.org/10.1101/2020.03.26.20044883
32. Hoffman T, Nissen K, Krambrich J, Rönnberg B, Akaberi D,
Esmaeilzadeh M, et al. Evaluation of a COVID-19 IgM and IgG rapid test; an
efficient tool for assessment of past exposure to SARS-CoV-2. Infect Ecol
Epidemiol. 2020 Abr;10(1):1754538. DOI:
https://doi.org/10.1080/20008686.2020.1754538
33. Pérez-García F, Pérez-Tanoira R, Romanyk J, Arroyo T, Gómez-Hurruz
P, Cuadros-González J. Rapid diagnosis of SARS-CoV-2 infection by detecting IgG
and IgM antibodies with an immunochromatographic device: a prospective
single-center study. medRxiv [Internet]. 2020 Abr 24; [Epub preprint]. DOI:
https://doi.org/10.1101/2020.04.11.20062158
34. Minteer C, Casanovas-Massana A, Li T, McDonald D, Wang L, Pan SH, et
al. Multi-site validation of a SARS-CoV-2 IgG/IgM rapid antibody detection kit.
medRxiv [Internet]. 2020 Mai 26; [Epub preprint]. DOI:
https://doi.org/10.1101/2020.05.25.20112227
35. Yangchun F. Optimize clinical laboratory diagnosis of COVID-19 from
suspect cases by likelihood ratio of SARS-CoV-2 IgM and IgG antibody. medRxiv
[Internet]. 2020 Abr 08; [Epub preprint]. DOI:
https://doi.org/10.1101/2020.04.07.20053660
36. Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Clinical
performance of SARS-CoV-2 IgG antibody tests and potential protective immunity.
medRxiv [Internet]. 2020 Mai 10; [Epub preprint]. DOI:
https://doi.org/10.1101/2020.05.08.085506
37. Qu J, Wu C, Li X, Zhang G, Jiang Z, Li X, et al. Profile of
immunoglobulin G and IgM antibodies against severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Out;71(16):2255-8. DOI:
https://doi.org/10.1093/cid/ciaa489
38. Capello F, Cipolla M, Cosco L, Gnasso A, Mancini R, Nichelatti M, et
al. The VivaDiag COVID-19 lgM/IgG rapid test for the screening and early
diagnosis of COVID-19 in patients with no clinical signs of the disease. Int
J
Endocr Metab Dis. 2020;6(1):1-4. DOI:
https://doi.org/10.16966/2380-548x.167
39. Xie J, Ding C, Li J, Wang Y, Guo H, Lu Z, et al. Characteristics of
patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody
test. J Med Virol. 2020 Abr;92(10:2004-10. DOI: https://doi.org/10.1002/jmv.25930
40. Ma H, Zeng W, He H, Zhao D, Jiang D, Zhou P, et al. Serum IgA, IgM,
and IgG responses in COVID-19. Cell Molec Immunol. 2020 Mai;17:773-5. DOI:
https://doi.org/10.1038/s41423-020-0474-z
1 . Pontifical Catholic University of the Rio
Grande do Sul, Postgraduate Program in Medicine and Health Sciences, Porto
Alegre, RS, Brazil.
2 . Mãe de Deus Health System, Hospital Mãe de Deus
Carlos Gomes, Porto Alegre, RS, Brazil.
Corresponding author:
Denis Souto Valente Rua Antônio Carlos Berta, 475/702, Jardim
Europa, Porto Alegre, RS, Brazil. Zip Code: 91340-020 E-mail:
denisvalentedr@gmail.com
Article received: July 05, 2020.
Article accepted: January 10, 2021.
Conflicts of interest: none