INTRODUCTION
Lipografting, a surgical technique known as lipomodeling or lipofilling, is an
essential alternative to breast reconstruction techniques after surgical
treatment of breast cancer1. This
procedure consists of the transfer of autologous fat to the breast, aiming to
restore the volume and revitalize and regenerate tissues damaged by adjuvant
treatments such as radiotherapy; or more recently described, for minor volume
adjustments conservative surgery for the treatment of breast cancer1,2. This regenerative potential is due to stem cells derived from
adipose tissue (ATSC), present in subcutaneous cellular tissue1-4. A practical way to obtain a donor area of ATSCs, followed by
preparation for injection in the receiving area, was described in the 1990s by
Coleman3,4. In this technique, the liposuction is
obtained using 3mm diameter needles coupled to 10mL syringes. After, the content
is centrifuged at 3000rpm for three minutes1,3,4. In practice,
there is significant variability of methods for obtaining liposuction and
separating the elements that compose it5.
ATSCs come from the same embryological lineage as bone marrow stem cells. Because
of the ease in obtaining surgery, they are considered an alternative source of
stem cells5-8. ATSCs can differentiate beyond
mesenchymal origin cells (adipocytes, myocytes, chondrocytes, and osteocytes)
in
non-mesenchymal cells, such as hepatocytes and neurons pancreatic cells,
endothelial cells, and cardiomyocytes7,8.
The viability of ATSCs may be related to the form used for the separation of
lipoaspirated elements4,5,7. Identifying management conditions that optimize the viability of
the content used is essential to improve lipografting as an adjuvant in mammary
reconstruction8,9. Despite being a widely used technique,
there is still questioning about the effect on ATSCs and adipocytes’ viability
after centrifugation; or after decanting of liposuction10,11.
OBJECTIVES
Therefore, the present study aimed to evaluate the cellular viability of isolated
ASCs from decanting and centrifugation at different speeds (500, 1000, and
3000rpm) and collection methods: Coleman, assisted conventional liposuction
(SAL), and associated with VASER (Vibration Amplification of Sound Energy at
Resonance).
METHODS
An experimental clinical study was conducted with samples of liposuction of
adipose tissue from patients older than 18 years, who agreed to participate in
the research and signed the free and informed consent form, and who underwent
liposuction procedures associated or not with lipofilling, performed in
hospitals in Chapecó - Santa Catarina, between June and July 2018. The patients
were randomly chosen during the proposed period for the collection of
lipoaspirated material.
To verify the influence of liposuction processing by decanting or centrifugation,
approximately 60mL of residual samples from each patient was evaluated,
subdivided into 4 Falcon® tubes of 15mL, one
subjected to decanting and each of the other three tubes subjected to
centrifugation at speeds other than 500, 1000 and 3000rpm (equivalent to 43,
173
and 1560g, respectively) (centrifuge with rotor: 11133 Buzzer,
Sigma®). For cell viability analysis, the ASCs
were treated with 3-bromide (4,5-dimetiltiazol-2-il)-2,5-diphenyltetrazolium
(MTT). In the control sample, 0.9% saline solution was added (SF 0.9%) in a 1:1
ratio and kept in decanting for 30 minutes12. The other samples were centrifuged at speeds of 500, 1000, and
3000 rpm, equivalent to 43, 173, and 1560g (considering that 2g is equivalent
to
100 rpm in the rotor centrifuge: 11133 Buzzer, Sigma®); for 3
minutes. Then, the centrifuged samples were washed with 0.9% SF and centrifuged
again for 10 minutes at 2000rpm. These four collagenase IA (C2674 -
Sigma®) samples were added to a concentration of 0.075% at
1:1 ratio, and samples were incubated in a water bath at 37ºC for 30 minutes
homogenization every 10 minutes12,13. For cell
viability analysis, ATSCs were treated with 3-bromide
(4,5-dimetiltiazol-2-il)-2,5-diphenyltetrazolium (TM).
From the 15mL Falcon® content, 200µL were aspirated from the pellet
bottom (lower portion of the tube) and 200µL from the intermediate region
and sent for cultivation in the 12-well plate, in triplicate, with the addition
of 2mL of the DMEM medium (Vitrocell-Embriolife ®), containing 10% SFB,
amphotericin and penicillin. The samples were grown in a greenhouse without CO2.
Each sample’s aliquot was removed and colored with Giemsa dye to evaluate the
cells’ morphology under optical microscopy. After 24 hours of cell culture, the
cells were submitted to the cell viability assay by 3-bromide
(4,5-dimetiltiazole-2-il)-2,5-diphenyltetrazozole (MTT). The plates were
centrifuged at 2500rpm for 15 minutes in a thermo-shaker centrifuge
(Agimaxx®.) The antinatant was discarded and 90
lµ0.9% at 37ºCand 10 µlof MTT reagent were added. He was in a dry
bath at 37ºC for 60 minutes. It was centrifuged again at 2500rpm for 15 minutes
and after discarding 70µL of each sample and adding 70µL
dimethylsuf(DMSO). The centrifugal was incubated for 10 minutes, the centrifugal
was repeated in the same conditions, and spectrophotometry of 560ηm12.14 was performed.
The data of cell culture of mesenchymal cells performed by the MTT technique were
statistically analyzed using the MEANS and UNIVARIATE procedures and, by
analysis of variance, using the statistical software SAS® (SAS
INSTITUTE, 2002). With the plate reading, a nonparametric ANOVA
study was performed by the NPAR1WAY procedure, and for comparison between the
means, the nonparametric test of the SAS®/Graphpad
Prism 6.0® software was adopted. The results of all analyses
were considered significant with p<0.05. This project was approved by the
Research Ethics Committee of Unochapecó, under Certificate of Presentation for
Ethical Appreciation (CAAE) 73059017.4.0000.0116 and opinion number
2,576,847.
RESULTS
During the period determined for the collection of lipoaspirated, it was possible
to evaluate 24 samples from six patients (five female and one male), with a
median age of 43 years. The choice that the lipoaspirated were of different
techniques was to verify whether there was the influence of the collection
methodology (Coleman, conventional suction-assisted liposuction and
VASER) according to the components’ separation of the
lipoaspirated. The cell viability rate was considerably higher in the samples
submitted to more intense centrifugations. The samples that went through
centrifugation at 3000rpm showed better results regarding cell viability in
ATSCs submitted to the MTT assay (0.309±0.08A). Centrifuged lipoaspirated
with 500 and 1000rpm showed relatively close ATSC viability rates
(0.246±0.12B and 0.253±0.08B, respectively). While the sample
washed with SF 0.9% and decanted for 30 minutes, followed by exposure to MTT,
presented the lowest viability rate of ATSCs (0.219±0.18AB) (Figure 1).
Figure 1 - Cell viability of samples decanted or centrifuged at 500, 1000,
and 3000 rpm and tested with MTT (n=6). Nonparametric ANOVA study of
samples in triplicate, in which columns correspond to the mean found
and bars to standard deviation.
Figure 1 - Cell viability of samples decanted or centrifuged at 500, 1000,
and 3000 rpm and tested with MTT (n=6). Nonparametric ANOVA study of
samples in triplicate, in which columns correspond to the mean found
and bars to standard deviation.
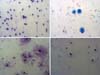
At the end of cell culture, samples stained with Giemsa dye and
observed under optical microscopy showed similar morphologies; no karyorrhexis,
a significant amount of binucleated cells, and no DNA evidence fragmentation
in
the different samples were observed (Figure 2).
Figure 2 - Histomorphological aspect of cell samples of lipoaspirated
patients submitted to decanting or centrifugation at different
speeds and stained by Giemsa. Decanting displayed at 400X.
Figure 2 - Histomorphological aspect of cell samples of lipoaspirated
patients submitted to decanting or centrifugation at different
speeds and stained by Giemsa. Decanting displayed at 400X.
DISCUSSION
Adipose cells’ viability may be related to several factors: characteristics of
the patient’s adipose tissue; extraction procedure; centrifuge methodology;
injection form of lipoaspirated; use of anesthetic solutions; topography of the
donor area; among others10,11,15. There is no
consensus on the most effective way to process fat (after aspiration of donor
areas) for better graft viability; several factors can influence the cells’
viability that makes up liposuction5,16,17.
For this research, we chose to include samples from different liposuction
techniques because the objective was to evaluate the impact of centrifugation
speed on cell viability, regardless of the technique used in the collection.
In
a study conducted with lipoaspirated residues from three different techniques
of
obtaining adipose cells, it was verified that SAL is the one that provides the
lowest amount of ATSCs18. On the other
hand, in an in-vivo study, in which collection sites were
treated with VASER, the authors concluded that similar results
were found in ASLC considering the quality of adipose cell retention5. In another experimental study, which
compared the technique of adipose tissue collection by SAL and the Coleman
technique, it was verified that the technique of aspiration with a syringe would
preserve the histological structure of adipocytes better than the ASC17.
The centrifugation process makes it possible to concentrate fat and provides a
more significant number of cells per milliliter, separating from liquefied fat
and blood cell components19. It was
reported that the ATSCs present in the pellet (after washing the centrifuge)
would have greater viability because there were no contaminants of the remains
of the blood cells, which would be a constant in samples submitted only to
decanting10. Centrifugation with
lower processing speeds and time may have the same cell compaction capacity
compared to speeds and times greater20,21. In our
study, the sample submitted only to decanting, and those that were centrifuged
at speeds of 500 rpm (43g) and 1000 rpm (173g) presented reduced cell viability
values concerning the sample of 3000 rpm. The fact that the samples submitted
to
centrifugation at lower velocities (500 and 1000 rpm) showed similar results
suggests that some factors such as cellular blood debris may have interfered
in
cell reproduction capacity. This was a limiter presented during the research;
because, with the established methodology, it was not possible to identify the
specific cause related to the lowest cell viability in samples that were not
centrifuged at a speed of 3000 rpm.
Considering the variable cell morphology in an experimental study, no cellular
changes were observed in optical microscopy examination at 3600 rpm
centrifugation for 10 seconds9. There was
no change in another experiment with lipoaspirated residues of eight patients,
centrifuged at velocities (400g, 700g, 1200g, 3000g, or 4200g) microscopic
morphology of cells16. While in another
study, with similar methodology and centrifugation velocities of 1500 and
3000rpm for 3 minutes, no cell membrane changes were described, including the
control sample (decanting)15.
In the present study, after 24-hour cultivation, it was possible to analyze the
morphology of cell samples stained with Giemsa, and no
morphological changes were observed between decanted and centrifuged samples
at
different speeds, but the cell viability evaluated by the MTT method was higher
in the centrifuged sample at 3000rpm for 3 minutes. These findings suggest that
both decanting and centrifugation velocities lower than 3000rpm may not
interfere with cells’ microscopic aspect, but the higher speed would be related
to maintaining cell multiplication capacity.
On the other hand, in experimental studies that used cell culture in rats, there
was an association of deleterious effects in cell samples submitted to higher
velocities when compared to those submitted to centrifugations with lower
velocities9,16. This difference in results may be
related to the form of cell culture methodology where most of the studies that
associated better cell viability with higher centrifugation velocities, as in
the present article, used cell culture in plates. In contrast, the other
researchers used the murine model.
CONCLUSION
Fat grafting is widely applicable, especially in breast reconstruction, whose
aesthetic and therapeutic effects are essential for the patient’s self-esteem,
contributing to the quality of life during treatment. In searching for
perfecting the technique, studies are carried out with the ASCs, who are mainly
responsible for the reconstructive potential. In this context, it was possible
to verify in this study that centrifugations with 3000 rpm for three minutes
results in maintaining the biological characteristics of the mesenchymal cells
of adipose tissue, necessary for cell colonization in the graft recipient areas.
This reinforces the Coleman technique’s applicability, which can be performed
in
surgical treatment services for breast cancer, as long as the surgeon is used
to
the methodology.
ACKNOWLEDGMENT
To all the components of the research group “Molecular Biology and Biotechnology
in Health” of Universidade Comunitária da Região de Chapecó (Unochapecó) and
to
the research group “Biological and Clinical Studies in Human Pathologies” of
Universidade Federal da Fronteira Sul (UFFS) Campus Chapecó/SC.
To the Hospital Regional do Oeste, Hospital da Criança Augusta Muller Bohner and
Hospital Unimed Chapecó for consenting the research. To surgeons: Cassiano
Furtado Beller, Fabio Portanova Barros, Gustavo Colonheze, Jorge Diego
Valentini, Liana Ortiz Streets Winkelmann, Rafael de Almeida Tirapelle and
Tainara Cassol.
REFERENCES
1. Fatah F, Lee M, Martin L, O'Donoghue JM, Sassoon EM, Weiler-Mithoff
M. Lipomodelling guidelines for breast surgery: joint guidelines from the
association of breast surgery, the British Association of Plastic,
Reconstructive and Aesthetic Surgeons, and the British Association of Aesthetic
Plastic Surgeons; 2012.
2. Biazus JV, Falcão CC, Parizotto AC, Stumpf CC, Cavalheiro JAC, Schuh
F, et al. Immediate reconstruction with autologous fat transfer following
breast-conserving surgery. Breast J. 2015 Jun;21(3):268-75.
3. Coleman SR. Long-term survival of fat transplants: controlled
demonstrations. Aesthetic Plast Surg. 1995 Out;19(5):421-5.
4. Coleman SR. Structural fat grafting. Aesthet Surg J. 1998
Set;18(5):386-8.
5. Fisher C, Grahovac TL, Schafer ME, Shippert RD, Marra KG, Rubin JP.
Comparison of harvest and processing techniques for fat grafting and adipose
stem cell isolation. Plast Reconstr Surg. 2013
Ago;132(2):351-61.
6. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al.
Multilineage cells from human adipose tissue: implications for cell-based
therapies. Tissue Eng. 2001 Abr;7(2):211-28.
7. Kumar V, Abbas AK, Fausto N. Adaptação, lesão e morte celular. In:
Kumar V, Abbas AK, Fausto N, eds. Robbins & Cotran - Patologia: bases
patológicas das doenças. 7ª ed. Rio de Janeiro: Elsevier; 2005. p.
4-47.
8. Moreno M, Silva AV. Caracterização da célula-tronco derivada do
tecido adiposo subcutâneo. Rev Bras Mastologia.
2012;22(4):138-43.
9. Hoareau L, Bencharif K, Girard AC, Gence L, Delarue P, Hulard O, et
al. Effect of centrifugation and washing on adipose graft viability: a new
method to improve graft efficiency. J Plast Reconstr Aesthet Surg. 2013
Mai;66(5):712-9.
10. Condé-Green A, Amorim NFG, Pitanguy I. Influence of decantation,
washing and centrifugation on adipocyte and mesenchymal stem cell content of
aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg.
2010 Ago;63(8):1375-81.
11. Gabriel A, Champaneria MC, Maxwell GP. Fat grafting and breast
reconstruction: tips for ensuring predictability. Gland Surg. 2015
Jun;4(3):232-43.
12. Yokomizo VMF, Benemond TMH, Bressan FF, Sangalli JR, Pieiri NCG,
Casals JB, e tal. Adipose tissue derived stem cells: isolation, in vitro culture
and potential uses in dermatology. Surg Cosmet Dermatol.
2011;3(1):55-9.
13. Ferreira RJ. Avaliação de diferentes métodos de detecção de
alterações celulares para triagem de células-tronco mesenquimais: estudo
pré-clínico (tese). Curitiba (PR): Universidade Federal do Paraná (UFPR);
2014.
14. Fukui M, Yamabe N, Zhu BT. Resveratrol attenuates the anticancer
efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur
J
Cancer. 2010 Jul;46(10):1882-91.
15. Kim IH, Yang JD, Lee DG, Chung HY, Cho BC. Evaluation of
centrifugation technique and effect of epinephrine on fat cell viability in
autologous fat injection. Aesthet Surg J. 2009
Jan/Fev;29(1):35-9.
16. Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K, Harii K, et al.
Influences of centrifugation on cells and tissues in liposuction aspirates:
optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr
Surg. 2008 Mar;121(3):1033-41.
17. Pu LLQ, Coleman SR, Cui X, Ferguson REH, Vasconez HC. Autologous fat
grafts harvested and refined by the Coleman technique: a comparative study.
Plast Reconstr Surg. 2008 Set;122(3):932-7.
18. Oedayrajsingh-Varma MJ, Van Ham SM, Knippenberg M, Helder MN,
Klein-Nulend J, Schouten TE, et al. Adipose tissue-derived mesenchymal stem cell
yield and growth characteristics are affected by the tissue-harvesting
procedure. Cytotherapy. 2006;8(2):166-77.
19. Ladeira PRS, Isaac C, Nakamura YM, Tutihashi RMC, Paggiaro AO,
Ferreira MC. Cultivo de células-tronco derivadas de tecido adiposo: uma análise
crítica. Rev Med (São Paulo). 2012 Dez;91(4):246.
20. Khoobehi K. Lipoenxertia na mama. In: Autor XX, ed. Contorno
corporal e lipoaspiração. Rio de Janeiro: Elsevier; 2014. p.
43-53.
21. Pulsfort AK, Wolter TP, Pallua N. The effect of centrifugal forces
on viability of adipocytes in centrifuged lipoaspirates. Ann Plast Surg.
2011;66(3):292-5.
1. Federal University of Fronteira Sul, Chapecó,
SC, Brazil.
2. Community University of the Region of Chapecó,
Medicine Course, Chapecó, SC, Brazil.
Corresponding author: Marcelo Moreno,
Área Rural, Área Rural de Chapecó, Rodovia SC 484, Km 02, Sala 210, Chapecó,
SC,
Brazil. Zip Code: 89815-899. E-mail:
marcelo.moreno@uffs.edu.br
Article received: March 10, 2020.
Article accepted: January 10, 2021.
Conflicts of interest: none