INTRODUCTION
The possibility of fat reinjection through cannula has transformed autologous fat
grafting into a convenient and effective tool to restore volume and subcutaneous contouring
in several clinical situations1,2. Currently, there are several technical possibilities for each step in the fat grafting
process, but there is no absolute and uniform consensus that can guarantee the best
efficacy and predictability in the integration of these grafts. Recently, two systematic
literature reviews revealed the current stage of evidence-based fat grafting, seeking
to elucidate the effects of different harvesting, processing, and reinjection methods
on fat graft integration rates. Interestingly, very few variables apply to the harvesting
method, including different types and sizes of cannulas, but they have always been
studied in different liposuction techniques3,4.
Fat transfer has been increasingly used in the gluteus and mammary contour, but the
volumetric limitation of liposuction as a method of material collection may represent
a formal impediment for many patients, especially in the post-bariatric population.
Large amount of weight loss promotes the emptying of subcutaneous cellular tissues,
which makes liposuction less effective in these cases, either due to established tissue
laxity or the small amount of remaining fat. Thus, the need to restore the best volume
in localized areas often results in technical difficulty or even the impossibility
to obtain sufficient fat through liposuction. Paradoxically, dermolipectomies performed
for suspension and body tightening in these patients invariably produce undesirable
discharge of viable fat tissue incorporated in surgical resections5.
To better obtain the autologous and injectable fat grafts from dermolipectomy surgeries,
we propose a new method for harvesting and processing adipose tissue through a shredder
that can convert almost all subcutaneous cell tissues into viable fat grafts6.
OBJECTIVE
The objective of this study was to establish an analytical comparison between the
characteristics and biological behavior of the new “fragmented fat” and the already established “liposuction” procedures, when used as a graft. In vitro and in vivo observations were designed to establish and evaluate parameters that objectively
prove the viability of the fragmented fat graft and support future studies and clinical
applications in humans.
METHODS
The scientific design of this project was properly protocoled and approved by the
ethics committees of the Botucatu School of Medicine - Paulista State University (UNESP)
for human (CAAE 73646217.7.0000.5411) and animal research (CEUA 1240/2017) between
September 2017 and March 2018.
An adult female patient, with significant weight loss after bariatric surgery, underwent
an anterior abdominal anchor dermolipectomy. The infra-umbilical region of her surgical
specimen used and medially divided into two parts. The left half received subcutaneous
infiltration of 400 mL Klein needle saline solution (t = tumescent), while the right
half was kept dry without any infiltration (d = dry). Each half was then vertically
divided into two parts, resulting in four different pieces that were subjected to
the two different graft harvesting techniques (Figure 1a). Both lateral portions received liposuction by syringe with 2-mm cannula (liposuction:
dry and tumescent [LAd and LAt, respectively]), while the two central portions had
their skin resected by scalpel and the fatty tissue divided into smaller pieces that
were then subjected to the new method of fragmentation (lipofragmentation: dry and
tumescent [FFd and FFt, respectively]).
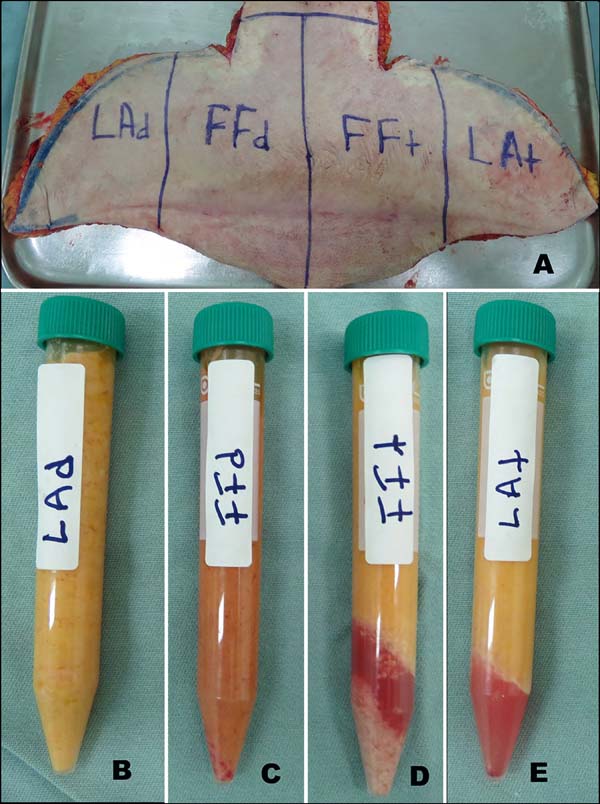
Figure 1 - A: Division of the surgical specimen from abdominal dermolipectomy; B-E: Samples of liposuction and dry lipofragmentation (LAd and FFd) and Infiltrate (LAt
and FFt).
Figure 1 - A: Division of the surgical specimen from abdominal dermolipectomy; B-E: Samples of liposuction and dry lipofragmentation (LAd and FFd) and Infiltrate (LAt
and FFt).
All the four samples obtained at the end were centrifuged (1,200 rpm for 3 minutes)
and distributed for in vivo and in vitro assays (Figures 1b, c, d, e). Each of the four adipose tissue samples (FFd, FFt, Lad, and LAt) was grouped and
labeled as a different color (blue, red, green, and black, respectively) to preserve
blind manipulation during the subsequent experimental phases until the results were
finally ready for recording and interpretation.
Fat Fragmentation
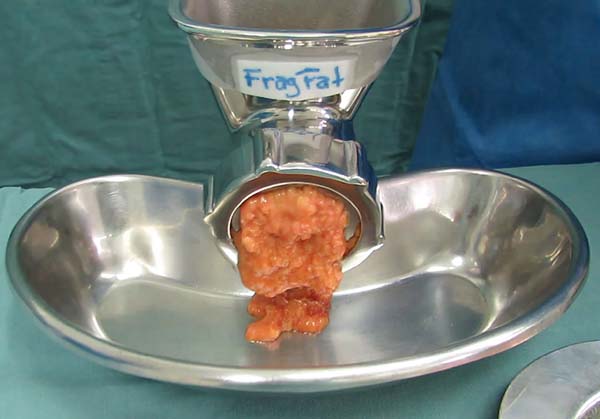
The proposed shredder is a hand-held apparatus (Figure 2), made of stainless steel (autoclavable) and designed to receive proximal portion
pieces of fatty tissue in a cylindrical chamber (Figure 2a), which are gradually carried by a central helical structure (Figure 2b) towards a distal cutting device consisting of a star-shaped rotational blade (Figure 2c) and a stationary multi-perforated plate (Figure 2d). Finally, a threaded ring holds the structures in place (Figure 2e). Using the crank handle, as the (skinless) pieces of fat are pushed forward by the
auger toward the distal cutting device, the tissue projects through plate holes and
is immediately and continuously sectioned into small fragments by the rotational blade.
The degree of fragmentation is determined by the hole diameter in the multi-perforated
plate, providing ideal consistency for lipoinjection according to different cannula
sizes and their internal diameters. In this experiment, we used a distal plate with
2-mm holes to obtain lipograft under conditions similar to liposuction that was collected
through a 2-mm diameter cannula (Figure 3).
Figure 2 - Fat Shredder Assembly. A: Cylindrical Chamber; B: Central Helical Rod; C: Star Cutting Blade; D: Multi-Perforated Distal Plate; E: Fixing Thread and F: Crank Mounted Device.
Figure 2 - Fat Shredder Assembly. A: Cylindrical Chamber; B: Central Helical Rod; C: Star Cutting Blade; D: Multi-Perforated Distal Plate; E: Fixing Thread and F: Crank Mounted Device.
Figure 3 - Shredder turning pieces of fat (without skin) into fat grafting.
Figure 3 - Shredder turning pieces of fat (without skin) into fat grafting.
In Vitro Study
All samples of fragmented fat (lipofragmentation) and liposuction were subjected to
routine histology including immunohistochemistry, viable cell count, flow cytometry,
and stromal vascular fraction cell culture. Samples in the in vitro study were made available through 1 ml syringes through a 2 mm cannula, in exactly
the same way as in the in vivo protocol.
Histological Assessment Each sample (3 g fat) was immediately fixed in 10% buffered formaldehyde, concentrated
by gravity filtration on porous paper, processed in different grades of alcohol and
xylol, embedded in paraffin, cut to 5 microns thick and subjected to H&E staining.
Immunohistochemical characterization was obtained by using the primary stromal progenitor
marker CD34. The histological slides were observed and compared by an experienced
pathologist seeking to analyze possible architectural tissue distortion, adipocyte
degeneration and immunohistochemical pattern.
Biological Assessment The protocol used for enzymatic dissociation was as detailed in previous publications7,8. Each of the 4 samples collected (10 g) was added to 30 mL of filtered Collagenase
solution (GibcoTM) using the following ingredient ratio: 3 mg of Collagenase type 2 per gram of tissue
and 1 mL of Hank’s balanced saline (GibcoTM) per mg Collagenase + 3.5% bovine serum albumin (Sigma Aldrich™). The 50 mL tubes,
filled with 10 g of tissue and 30 mL of filtered Collagenase solution, were submerged
in a 37 °C water bath for 30 min under constant agitation until the solution became
homogeneous. The samples were subjected to double-layer gauze filtration and re-centrifuged
at 120 rpm for 10 minutes, the digested fat fractions remained in the supernatant
while the stromal pellet condensed at the bottom. The viability of the suspended cells
was evaluated by the Trypan Blue9,10 (Sigma Aldrich™) technique. The culture of enzymatically dissociated cells and phenotyped
by flow cytometry aimed to evaluate the prevalence of possible and distinct progenitor
populations, comparing their biological behavior among groups11.
In this study, 104 cells/mL were seeded in 6-well plates containing DMEM F12 culture medium (Gibco™)
supplemented with streptomycin, penicillin, amphotericin B (Gibco™), and 20% fetal
bovine serum. Cells were monitored daily by inverted phase contrast microscope (Axiovert
A1 - Carl Zeiss™).
According to a joint definition of the International Federation for Adipose Therapeutics
and Science (IFATS) and the International Society for Cellular Therapy (ISCT)12, characterization of mesenchymal stem cells by flow cytometry requires a minimal
phenotypic expression pattern that provides positivity for some markers (we used CD90
and CD105) and negativity for others (we use dCD34 and CD45)12. In this study, the evaluation of surface molecular expression was performed using
FACScalibur BD equipment and Cell Quest Pro software using anti-human FITC CD45 (368507
Biolegend), anti-human PE CD34 (343505 Biolegend), FITC anti-human CD90 (Thy 1) (328107
Biolegend), and FITC anti-human CD105 (323203 Biolegend), with their respective control
isotopes.
In Vivo study
In order to compare the integration of lipograft from liposuction and lipofragmentation
in both dry and infiltrated groups, the different samples obtained were labeled with
different colors (blue, red, green, and black) and distributed in 1 mL syringes, which
were immediately taken from the operating room to the experimental laboratory, where
they were injected into the dorsal subcutaneous tissue of 10 Wistar rats under anesthesia
and sterile conditions. Two small punctures were positioned in the rats’ dorsal midline
and using 2 mm diameter cannulas 1 mL of each sample (FFd, LAd, FFt, and LAt) were
re-injected subcutaneously, grafting them equally on each of the quadrants in a clockwise
rotation protocol of sample placement for each new animal. The rats were marked by
numbers (ear method) and each injected graft sample was properly recorded by its color
and location. The animals were kept under standard environmental conditions and daily
received ventral subcutaneous injections of cyclosporine (2 mg/kg) to reduce the immune
response against xenograft13. After 6 weeks, all rats were sacrificed and the remaining fat from the grafts was
surgically removed for a comparative evaluation of mass and volume, as well as routine
histological preparation for H&E and Masson’s trichrome stained slides. Each slide
had areas randomly chosen for blind histological evaluation, seeking to classify the
presence of intact fat cells, cysts and vacuoles, inflammation (lymphocyte and macrophage
infiltration), fibrosis, and neovascularization (capillary density). The presence
of each of these variables were graded on a scale from 0 to 5, where: 0, absent; 1,
Minimum; 2, Minimum to moderate; 3, Moderate; 4, Moderate to extensive; 5, Extended14.
RESULTS
The fragmentation process was effective in transforming the pieces of dermolipectomy
fat tissue into an injectable fat grafting texture (Figure 3). All samples of fat material obtained by liposuction and lipofragmentation were
subjected to centrifugation resulting equally in a discreet superior oily layer. Both
samples from the infiltrated group (LAt and FFt) formed a liquid layer after centrifugation,
which as well as the oily layer were removed from the material to be studied, as described
by Coleman15,16. The infiltrated fragmented fat presented an inferior visible and sedimented layer
(pellet) that was not observed in the infiltrated liposuction and was not included
in the study material. The dry group material, liposuction and lipofragmentation,
had no liquid layer or lower pellet (Figures 1b, c, d, e).
Histological Assessment and Immuno-histochemistry
A blind analysis of both dry and infiltrated groups of both liposuction and lipofragmentation
material showed similar histological structural characteristics regardin g tissue
architecture and cellular integrity. Immunohistochemistry showed large number of CD34
labeled cells widely distributed between both liposuction and lipofragmentation tissues.
The histology of the sedimented material obtained after centrifugation of the infiltrated
lipofragmentation (pellet) revealed adipocytes amid a large amount of vascular and
stromal matrix, demonstrating high CD34 labeled cells (Figure 4).
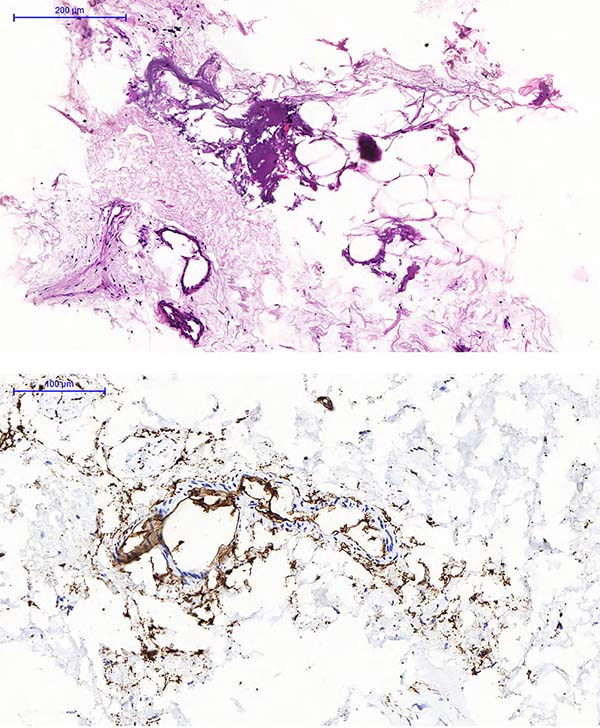
Figure 4 - Material of Pellet formed in the centrifugation of lipofragmentation Infiltrate (FFt). A. HE staining; B. Immunohistochemistry staining with a CD34 marker.
Figure 4 - Material of Pellet formed in the centrifugation of lipofragmentation Infiltrate (FFt). A. HE staining; B. Immunohistochemistry staining with a CD34 marker.
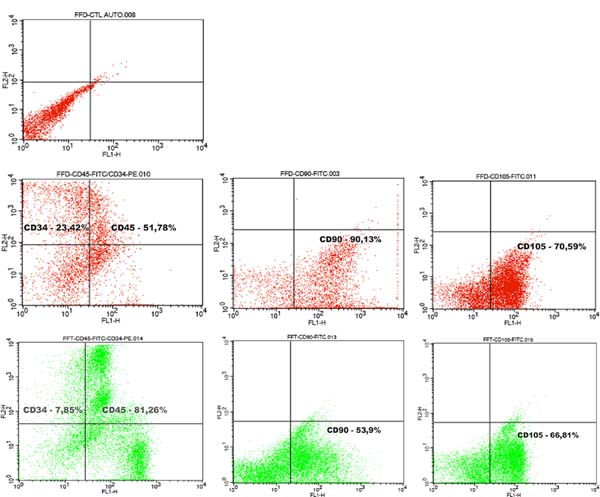
Counting of Viable Cells and Flow Cytometry
Although there was a significant difference in the number of nucleated cells among
samples, their viability did not vary significantly (Table 1). Trypan Blue test analysis showed no statistically significant difference in cell
viability between liposuction and lipofragmentation samples in both the dry and infiltrated
groups. Immunophenotyping of samples by flow cytometry showed that in both the dry
and infiltrated groups, liposuction and lipofragmentation also showed similar behavior
with the markers used in the study (Figures 5 and 6). The cells’ analytical distribution by each marker in the different preparations
showed no statistically significant difference (Table 1).
Table 1 - Immunophenotyping of progenitor cells by flow cytometry with positive and negative
markers and the mean fluorescence intensity in different samples.
| Mean of Fluorescence Intensity (%) |
| |
Ctle Auto |
Ctke ISO |
CD45-FITC |
CD34-PE |
CD90-FITC |
CD105-FITC |
∑ Number of cells analyzed |
| FFd |
6 |
11.91 |
52.98 |
51.81 |
92.22 |
69.09 |
11.095 |
| FFt |
5.07 |
6.64 |
84.12 |
57.67 |
55.98 |
65.42 |
20.000 |
| LAd |
2.61 |
3.4 |
61.39 |
63.78 |
51.52 |
37.23 |
20.000 |
| LAt |
6.09 |
4.21 |
56.14 |
78.33 |
70.67 |
68.68 |
20.000 |
Table 1 - Immunophenotyping of progenitor cells by flow cytometry with positive and negative
markers and the mean fluorescence intensity in different samples.
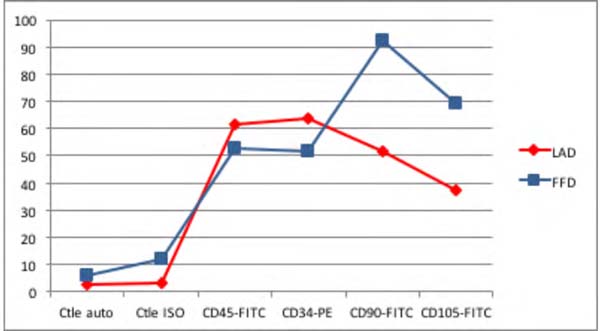
Figure 5 - Dry Group: Immunophenotype comparing the samples of liposuction (LAd) and lipofragmentation
(FFd).
Figure 5 - Dry Group: Immunophenotype comparing the samples of liposuction (LAd) and lipofragmentation
(FFd).
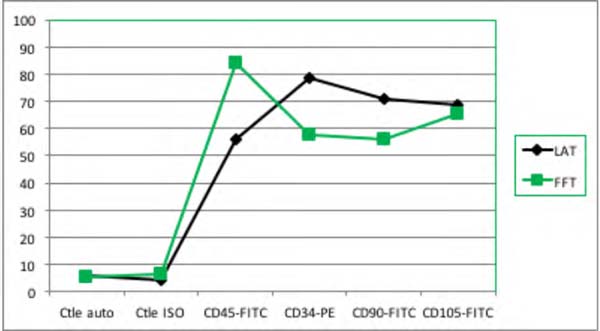
Figure 6 - Infiltrate Group: Immunophenotype comparing the samples of liposuction (LAt) and lipofragmentation.
Figure 6 - Infiltrate Group: Immunophenotype comparing the samples of liposuction (LAt) and lipofragmentation.
Among Dry group samples, the lipofragmentation technique showed the best performance
for markers CD90 and CD105, obtaining a higher quantification of mesenchymal stem
cells. On the other hand, in the infiltrated group, the lipofragmentation showed better
performance for CD45 markers, while liposuction was better for CD34. Figure 7 shows the representation of immuno-phenotypic analysis for AD-MSC surface markers
in the different samples.
Figure 7 - Representation of immunophenotype (Flow cytometry) conducted in samples with panel
of markers: CD34, CD45, CD90, and CD105.
Figure 7 - Representation of immunophenotype (Flow cytometry) conducted in samples with panel
of markers: CD34, CD45, CD90, and CD105.
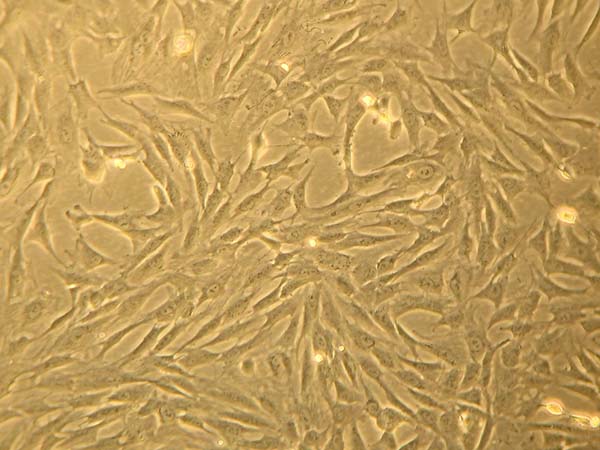
Fat-derived Stem Cell Culture
Liposuction and lipofragmentation samples in both the dry and infiltrated groups showed
similar parameters in cell culture, with standard regular expansion. The adhesion
rate at 72 hours was 25% for FFd samples, which differed significantly from the other
samples that reached 10%. Likewise, 100% cell confluence also occurred earlier in
the FFd sample. Furthermore, the final appearance of fibroblastic populations did
not differ between studied samples (Figure 8).
Figure 8 - Petri dish of culture of lipofragmention dry (FFd). Appearance at 30 days.
Figure 8 - Petri dish of culture of lipofragmention dry (FFd). Appearance at 30 days.
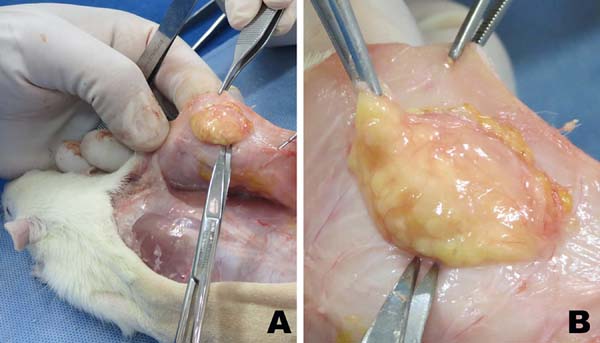
Lipograft Integration - Mass and Volume Analysis
Six weeks after fat grafting, all the 10 rats were sacrificed and had their remaining
grafts in the dorsal regions surgically removed (Figure 9). Student’s t-tests for independent samples were used for analysis of mass and volume
of obtained pieces, to compare the retention and behavior of lipofragmentation and
liposuction in both dry (FFd X LAd) and infiltrated (FFt X LAt) groups. The mean and
standard deviation (SAS® for Windows v. 9.3) were calculated with the production of graphic illustration (Statistica® for Windows v. 10) with a significance level set at 5% (p <0.05).
Figure 9 - A: Wistar rat dorsal grafts at 6 weeks. B. Xenograft removal in detail.
Figure 9 - A: Wistar rat dorsal grafts at 6 weeks. B. Xenograft removal in detail.
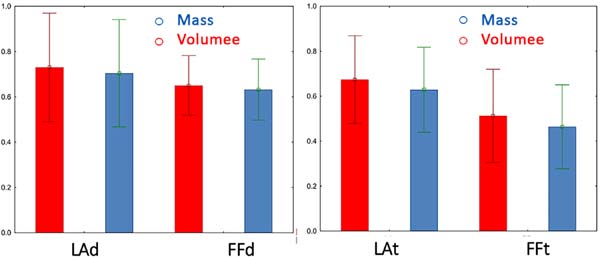
DRY: Lipofragmentation X Liposuction In the group where the fat was harvested and processed without previous infiltration,
the mean retained volume of lipofragmentation was 0.65 ± 0.13, while that in the liposuction
group was 0.73 ± 0.24 (p=0.37). In contrast, the mean retained mass after lipofragmentation was 0.63 ± 0.13,
while that after liposuction was 0.70 ± 0.24 (p=0.41). Hence, there were no statistically significant differences in the final mass
and volume of grafts by dry lipofragmented and liposuction grafts (Figure 10).
INFILTRATE: Lipofragmentation X Liposuction The mean volume retained in grafts of the harvested
group after infiltration of the pieces after lipofragmentation was 0.51 + 0.21, while
that after liposuction was 0.67 + 0.20 (p=0.09). The mean retained mass after lipofragmentation was 0.46 + 0.19, while that
after liposuction was 0.63 + 0.19 (p=0.06). There was no statistically significant difference in the final mass and volume
retention rates of lipografts obtained by infiltrate lipofragmentation and liposuction
(Figure 10).
Figure 10 - Comparison of Mass and Volume of different grafts retained after six weeks, in both
dry group (LAd and FFd) and infiltrate group (LAt and FFt).
Figure 10 - Comparison of Mass and Volume of different grafts retained after six weeks, in both
dry group (LAd and FFd) and infiltrate group (LAt and FFt).
Lipograft Integration - Histological Analysis
A widescreen digital scanner equipment (3DHistech®) and Pannoramic Viewer for Windows v. 1.15 software were used to handle slides and
to aid in grading histological variables on H&E staining, except for fibrosis that
was accessed by Masson’s trichrome staining. Data statistical treatment obtained by
microscopic assessment of grafts retained in the dry group (FFd and LAd) showed no
difference in relation to fibrosis inflammation and neovascularization. However, dry
liposuction showed a higher degree of cellular integrity and lower degree of cysts
and vacuoles when compared to dry lipofragmentation. In the infiltrated group (FFt
and LAt), there was no difference in cell integrity, inflammation and neovascularization,
but lipofragmentation had fewer cysts or vacuoles and higher fibrosis compared to
liposuction. The results were shown as mean and standard deviation (Student’s t-test),
and p < 0.05 was considered for statistical significance (Table 2).
Table 2 - Comparative representation of values obtained in the histological evaluation of grafts
removed from rats (liposuction X lipofragmentation) in both dry and infiltrated groups
| |
Lipofragmentation Dry |
Liposuction Dry |
p |
| Mean |
Standard Deviation |
Mean |
Standard Deviation |
| Cellular Integrity |
1.8 |
0.63 |
3.0 |
0.94 |
0.0036 |
| Cysts/Vacuoles |
3.4 |
1.26 |
2.3 |
0.82 |
0.0333 |
| Inflammation |
2.7 |
0.67 |
2.3 |
0.82 |
0.2502 |
| Fibrosis |
2.1 |
0.74 |
1.9 |
0.74 |
0.5520 |
| Neovascularization |
2.1 |
0.88 |
2.2 |
1.03 |
0.8180 |
| |
|
|
|
|
|
| |
Lipofragmentation Infiltrate |
Liposuction Infiltrate |
p |
| Mean |
Standard Deviation |
Mean |
Standard Deviation |
| Cellular Integrity |
2.3 |
1.16 |
3.4 |
1.26 |
0.0577 |
| Cysts/Vacuoles |
3.2 |
0.79 |
1.8 |
0.79 |
0.0009 |
| Inflammation |
2.3 |
0.67 |
2.3 |
0.95 |
1.000 |
| Fibrosis |
1.9 |
1.2 |
3.1 |
0.88 |
0.0197 |
| Neovascularization |
2.0 |
0.47 |
2.4 |
0.97 |
0.2603 |
Table 2 - Comparative representation of values obtained in the histological evaluation of grafts
removed from rats (liposuction X lipofragmentation) in both dry and infiltrated groups
DISCUSSION
Since we are proposing a new methodology for graft harvesting and processing, the
scope of this experiment was to promote a structural and biological comparison between
the new “lipofragmentation” and the already recognized “liposuction” procedures. We
used the same donor area from a single person to eliminate interpersonal variability
in fat quality regarding its regenerative potential and subsequent fat grafting retention17. The skin resection with scalpel and the fat piece division into smaller pieces allowed
the subcutaneous full thickness to be used, including fat layers and connective tissue,
the latter usually not incorporated by conventional liposuction.
The shredder can use different sizes in the distal multi-perforated plate holes (we
used 2 mm diameter holes) to allow injection of the collected material through different
cannula sizes, according to different areas to be grafted such as face, breasts and
buttocks.
Although this was not a specific target of our method, it seems quite clear that lipofragmentation
requires less time and physical effort on the part of the surgeon, obtaining higher
amounts of fat than traditional liposuction, especially if we consider the post-bariatric
patient with sparse and hollow fat layers.
Tumescent infiltration was initially developed to improve the liposuction18 technique, but some studies have shown its potential adverse effects that may impact
the better integration of fat grafts19-23. As this is a reality in liposuction, we chose to include a group of tumescent infiltration
because there are reports that such infiltration could negatively or positively impact
graft biology and viability3, which was not observed in the present study. We consider as an intrinsic bias of
our method the fact that in the initial surgical specimen demarcation to be a source
for graft harvesting, the paraumbilical (median) regions contain more perforating
vessels and a denser fascia of Scarpa than the lateral areas of the abdomen. Samples
from medial regions may present denser cell suspensions than lateral areas, which
could in theory justify some difference in the composition and biology of extracted
grafts.
Considering that we do not have the so-called nude mice in our laboratories, we used
Wistar rats with daily cyclosporine application to promote desirable immunosuppression
as previously described by Ferreira et al24. We recognize the athymic rat as the most appropriate model for this type of fatty
xenograft research and look forward to using them in future trials. Hence, reducing
the amount of fat injected as well as extending graft integration time to 12 weeks25.
The observation of our in vitro and in vivo assays has shown that lipofragmentation
had structural and biological characteristics very similar to those of liposuction.
Recently, evidence of lipografts’ dynamic and regenerative nature has emerged from
different areas of plastic surgery as well as cellular and molecular biology, including
the possibility of direct transplantation of fat-derived stem cells including their
biological niche, even without their formal isolation in cultivation medium not only
to increase graft retention but also as distinct sources of cell therapy26,27. Flow cytometry with a surface panel of specific markers, as well as cell viability
tests helped to distinguish and confirm possible stromal vascular fraction populations
present in lipofragmented material without statistical difference in relation to liposuction
material, showing that the new method preserved and did not cause significant damage
to these important structures.
The mechanisms involved in the fragmentation process probably incorporates more than
just fatty cellular content, but also its scaffolds. Following centrifugation, the
infiltrated lipofragmentation formed an evident lower pellet consisting of mature
adipocytes surrounded by a rich stromal web with blood vessels and nucleated cells,
including progenitor stem cells. The fact that we did not include this sedimented
material in the infiltrated lipofragmentation samples could, in theory, justify its
slightly lower integration after 6 weeks. Although that stromal tissue was not separated
and visually identified in the dry lipofragmentation samples, it is quite reasonable
to imagine that it should have been incorporated into the FFd samples, which had the
best integration rates. Subsequent investigations will be crucial in determining the
real potential of lipofragmentation stromal content in the graft enrichment and probable
clinical applications. Future studies with this material may also explore some modern
concepts of improvement of tissue healing as well as site-specific regenerative medicine
by using the perivascular scaffold probably incorporated in this new form of fat grafting.
CONCLUSION
The methods used showed that the shredder device was able to transform the subcutaneous
layers removed in a conventional dermolipectomy into a viable lipograft, and this
new type of Fragmented lipograft behaved with similar biological characteristics to
those exhibited by the traditional syringe liposuction. Although preliminary, our
results may support further studies in humans to extend lipofragmentation as an alternative
to fatty grafting as well as possible clinical applications for wound healing and
regenerative medicine.
COLLABORATIONS
|
FHM
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Data Curation, Final manuscript approval, Formal Analysis, Investigation, Methodology,
Project Administration, Realization of operations and/or trials, Resources, Supervision,
Validation, Visualization, Writing - Original Draft Preparation, Writing - Review
& Editing
|
|
FV
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Final manuscript approval, Methodology, Project Administration, Supervision, Visualization,
Writing - Original Draft Preparation, Writing - Review & Editing
|
|
ED
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Formal Analysis, Investigation, Methodology, Project Administration, Realization of
operations and/or trials, Resources, Supervision, Writing - Original Draft Preparation,
Writing - Review & Editing
|
|
MACD
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Final manuscript approval, Investigation, Methodology, Project Administration, Realization
of operations and/or trials, Supervision, Writing - Original Draft Preparation
|
|
MAG
|
Conception and design study, Conceptualization, Data Curation, Formal Analysis, Investigation,
Methodology, Realization of operations and/or trials
|
|
JMG
|
Data Curation, Investigation, Methodology, Realization of operations and/or trials
|
|
RRR
|
Data Curation, Investigation, Methodology, Realization of operations and/or trials
|
|
HCN
|
Data Curation, Investigation, Methodology, Realization of operations and/or trials
|
REFERENCES
1. Illouz YG. The fat cell “graft”: a new technique to fill depressions. Plast Reconstr
Surg. 1986;78:122-123. DOI: https://doi.org/10.1097/00006534-198607000-00028
2. Bircoll M, Novack BH. Autologous fat transplantation employing liposuction techniques.
Ann Plast Surg. 1987;18:327-329. DOI: https://doi.org/10.1097/00000637-198704000-00011
3. Strong AL, Cederna PS, Rubin JP, Coleman SR, Levi B. The current state of fat grafting:
a review of harvesting, processing, and injection techniques. Plast Reconstr Surg.
2015 Oct;136(4):897-912. PMID: 26086386 DOI: https://doi.org/10.1097/PRS.0000000000001590
4. Sinno S, Wilson S, Brownstone N, Levine SM. Current thoughts on fat grafting: using
the evidence to determine fact or fiction. Plast Reconstr Surg. 2016 Mar;137(3):818-824.
DOI: https://doi.org/10.1097/01.prs.0000479966.52477.8b
5. Mendes FH, Viterbo F. Abdominoplasty after massive weight loss. In: Avelar JM, ed.
New concepts on abdominoplasties and further aplications. New York: Springer; 2016.
p. 356-388.
6. Mendes FH, Viterbo F, Deffunne E, et al. Fragmented fat: a new method for harvesting
and processing of lipograft. J Plast Reconstr Aesthet Surg. 2019 Jun;72(6):1030-1048.
PMID: 30824381 DOI: https://doi.org/10.1016/j.bjps.2019.02.001
7. Zuk PA, Zhu M, Muzuno H, et al. Multilineage cells from human adipose tissue: implications
for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211-28. DOI: https://doi.org/10.1089/107632701300062859
8. Carvalho AM, Alves AL, Golim MA, et al. Isolation and immunophenotypic characterization
of mesenchymal stem cells derived from equine species adipose tissue. Vet Immunol
Immunopathol. 2009 Dec;132(2-4):303-6. DOI: https://doi.org/10.1016/j.vetimm.2009.06.014
9. Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2015
Nov;111(1):A3.B.1-3. DOI: 10.1002/0471142735.ima03bs111
10. Boschert MT, Beckert BW, Puckett CL, Concannon MJ. Analysis of lipocyte viability
after liposuction. Plast Reconstr Surg. 2002 Feb;109(2):761-765;discussion:766-7.
DOI: https://doi.org/10.1097/00006534-200202000-00055
11. Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation and
characterization of human mesnchymal stem cells. Cytometry A. 2018 Jan;93(1):19-31.
DOI: https://doi.org/10.1002/cyto.a.23242
12. Bourin P, Brunnell BA, Castella L, et al. Stromal cells from the adipose tissue-derived
stromal vascular fraction and culture expanded adipose tissue-derived stromal stem
cells: a joint statement of the International Federation for Adipose Therapeutics
and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy.
2013 Jun;15(6):641-8. DOI: https://doi.org/10.1016/j.jcyt.2013.02.006
13. Fritz WD, Swartz WM, Rose S, et al. Limb allografts in rats immunosuppressed with
cyclosporine A. Ann Surg. 1984;199(2):211-5.
14. Li K, Gao J, Zhang Z, et al. Selection of donor site for fat grafting and cell isolation.
Aesthetic Plast Surg. 2013 Feb;37(1):153-8. PMID: 23232729 DOI: https://doi.org/10.1007/s00266-012-9991-1
15. Coleman SR. Long-term survival of fat transplants: Controlled demonstrations. PMID:
8526158 Aesthetic Plast Surg. 1995;19(5):421-425.
16. Coleman SR. Structural fat grafting: More than a permanent filler. Plast Reconstr
Surg. 2006 Sep;118(3 Suppl):108S-120S. DOI: https://doi.org/10.1097/01.prs.0000234610.81672.e7
17. Fisher C, Grahovac TL, Schafer ME, Shippert RD, Marra KG, Rubin JP. Comparison of
harvest and processing techniques for fat grafting and adipose stem cell isolation.
Plast Reconstr Surg. 2013 Aug;132(2):351-61. PMID: 23584621 DOI: https://doi.org/10.1097/PRS.0b013e3182958796
18. Klein JA. Tumescent technique for local anesthesia improves safety in large- volume
liposuction. Plast Reconstr Surg. 1993 Nov; 92(6):1085-1098. DOI: https://doi.org/10.1097/00006534-199311000-00014
19. Agostini T, Lazzeri D, Pini A, et al. Wet and dry techniques for structural fat graft
harvesting. Plast Reconstr Surg. 2012 Aug;130(2):331e-339e. PMID: 22495217 DOI: https://doi.org/10.1097/PRS.0b013e3182589f76
20. Moore Junior JH, Kolaczynski JW, Morales LM, et al. Viability of fat obtained by syringe
suction lipectomy: Effects of local anesthesia with lidocaine. Aesthetic Plast Surg.
1995;19:335-339. DOI: https://doi.org/10.1007/BF00451659
21. Livaoğlu M, Buruk CK, Uraloğlu M, et al. Effects of lidocaine plus epinephrine and
PMID: 22777468 prilocaine on autologous fat graft survival. J Craniofac Surg. 2012
Jul;23(4):1015-8.
22. Shoshani O, Berger J, Fodor L, et al. The effect of lidocaine and adrenaline on the
viability of injected adipose tissue: An experimental study in nude mice. J Drugs
Dermatol. 2005 May/Jun;4(3):311-6. PMID: 15898286
23. Keck M, Zeyda M, Gollinger K, et al. Local anesthetics have a major impact on viability
of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg. 2010
Nov;126(5):1500-5. PMID: 21042106 DOI: https://doi.org/10.1097/PRS.0b013e3181ef8beb
24. Ferreira LM, Borsanyi JP, Bemhain P, et al. Rat limb allotransplantation: Efficacy
of subtherapeutic dose combination immunotherapy with cyclosporine and RS-61443. Rev
Hosp São Paulo Esc Paul Med. 1995;6(1-2):15-9.
25. Kato H, Mineda K, Eto H, et al. Degeneration, regeneration, and cicatrization after
fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr
Surg. 2014 Mar;133(3):303e-313e. PMID: 24572875
26. Galie M, Pignatti M, Scambi I, Sbarbati A, Rigotti G. Comparison of different centrifugation
protocols for the best yield of adipose-derived stromal cells from lipoaspirates.
Plast Reconstr Surg. 2008 Dec;122(6):233e-234e. PMID: 19050511 DOI: https://doi.org/10.1097/PRS.0b013e31818d2326
27. Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage
by lipoaspirate transplant: a healing process mediated by adipose- derived adult stem
cells. Plast Reconstr Surg. 2007 Apr;119(5):1409-22;discussion:1423-4. DOI: https://doi.org/10.1097/01.prs.0000256047.47909.71
1. Faculdade de Medicina de Botucatu, Universidade Estadual Paulista, Botucatu, SP,
Brazil.
Corresponding author: Flavio Henrique Mendes Rua Claudio Manoel da Costa, Nº 65, Jardim Ariano, Lins, SP, Brazil. Zip Code: 16400-464.
E-mail: mendesmd@fhmendes.com.br
Article received: July 16, 2019.
Article accepted: August 2, 2019.
Conflicts of interest: none.
Institution: Faculdade de Medicina de Botucatu, Universidade Estadual Paulista, Botucatu,
SP, Brazil.