INTRODUCTION
Endometriosis is characterized by the presence of functional endometrial tissue (endometrial
glands and stroma) outside the uterine cavity and uterine musculature; this tissue
usually responds to hormonal stimulation1,2,3,4. The uterine cavity is the site most commonly affected by endometriosis. Nonetheless,
few cases of extrapelvic endometriotic implants have been described in the literature5,6.
The diagnosis of endometriosis should be considered in women with a clinical history
of dysmenorrhea, chronic acyclic pelvic pain, dyspareunia, cyclic intestinal and urinary
symptoms, and infertility7. Confirmation of this disease is only possible by histopathological analysis of the
tissue fragments obtained by invasive procedures because no adequate clinical marker
is currently available1.
Endometriosis can occur in many different locations, including the vagina, vulva,
cervix, perineum, inguinal canal, urinary tract, gastrointestinal tract, pulmonary
tract, lungs, limbs, and skin. It can be considered as a nonspecific tissue in the
groin by plastic surgeons or as an inguinal hernia by general surgeons2.
After lesion removal, the plastic surgeon’s challenge is repairing the open area,
restoring function, and achieving the best aesthetic results. Several strategies can
be used to close the lesion depending on lesion location and size and patient status8.
OBJECTIVE
This study describes a viable option for repairing the inguinal region and labia majora
using a lower abdominal flap as one of the vertices of a Z-plasty with good aesthetic
results.
METHODS
The analyzed data were collected from medical records, patient interviews, photographic
records, and a literature review. This patient was treated in Brasília, Federal District,
Brazil, between September 2013 and February 2016. Patient privacy was protected, and
the patient authorized the publication of the data and signed an informed consent
form. The study adhered to the principles of the Declaration of Helsinki.
RESULTS
J.A.K, a 30-year-old nulliparous female patient with a body mass index of 29 kg/m2 and gynecological follow-up, presented with a 3-year history of dysmenorrhea, pain,
and paresthesia in the right inguinal region, functional limitation during the menstrual
period, and a firm nodule in the labia majora with scars from two surgical endometriosis
resections. Bleeding occurred monthly through the umbilicus. The gynecology team presented
surgical planning involving the endoscopic resection of cavitary lesions, inguinal
canal content, nodules in the right labia majora with margins, umbilicus, and part
of the aponeurosis. Nonetheless, larger defects requiring surgical reconstruction
developed after the excision of lesions in the inguinal region, right labia majora,
and umbilicus.
Surgical planning included repair of the abdominal wall in the periumbilical region,
plication of the aponeurosis of the rectus abdominis muscles 5 cm above and 5 cm below
the umbilical scar, closure of the abdominal wall defect, construction of a new umbilicus,
and suturing between the dermis and the aponeurosis of the rectus abdominis (the umbilicus
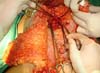
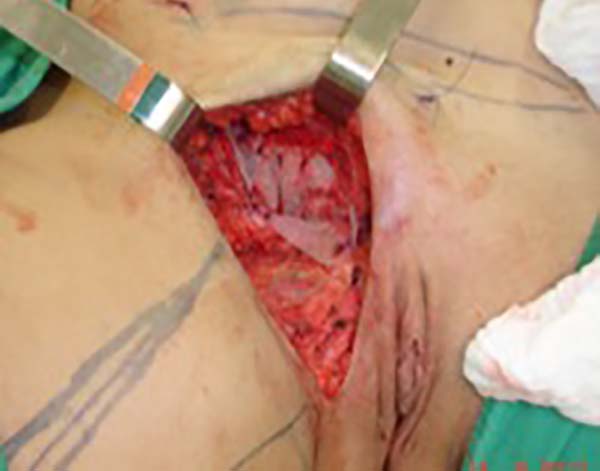
was resected en bloc) (Figure 1). In the inguinal region, the integumentary defect affected more than 70% of the
right labia majora and the thigh root region with exposure of the inguinal canal and
communication of the uterine cavity with the external environment because the endometriosis
impacted the region from the uterine cavity to the labia majora (apparently along
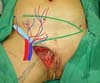
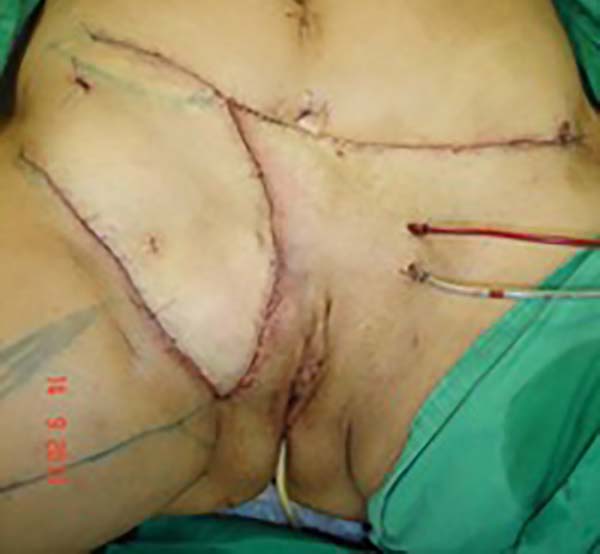
the funiculus spermaticus) (Figure 2). The surgical planning was based on recurrence potential, minimizing donor area
damage to prevent an inguinal hernia due to the defect, and the use of local flaps
to increase vascularization (Figure 3).
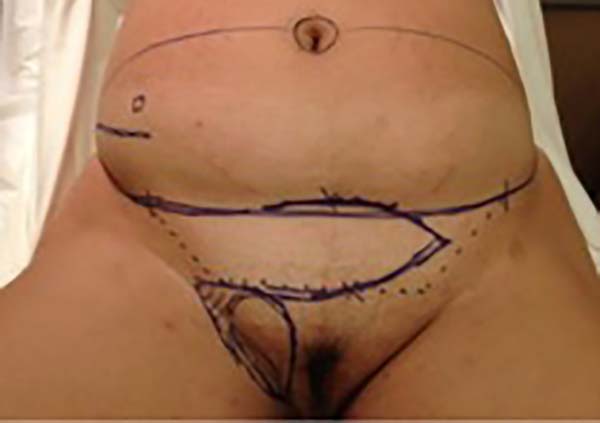
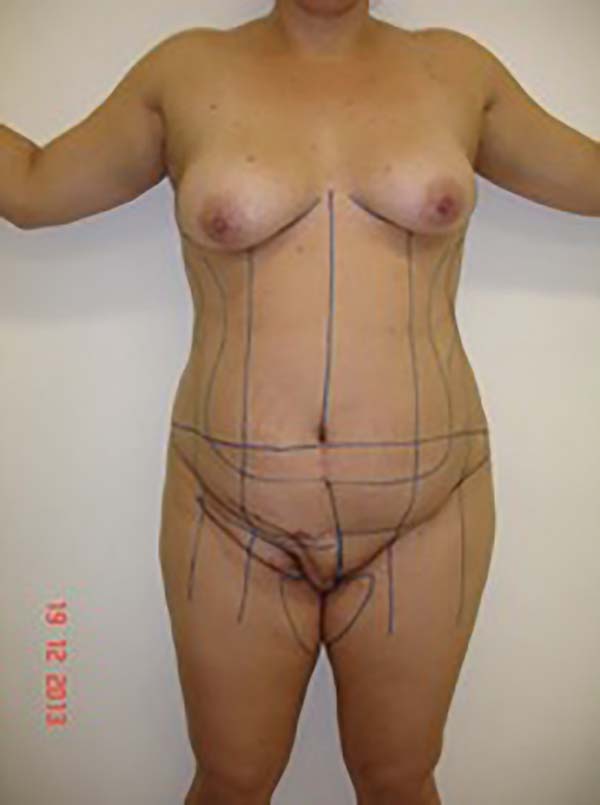
Figure 1 - Initial patient assessment and the possible use of a surgical flap (may be larger
depending on the defect).
Figure 1 - Initial patient assessment and the possible use of a surgical flap (may be larger
depending on the defect).
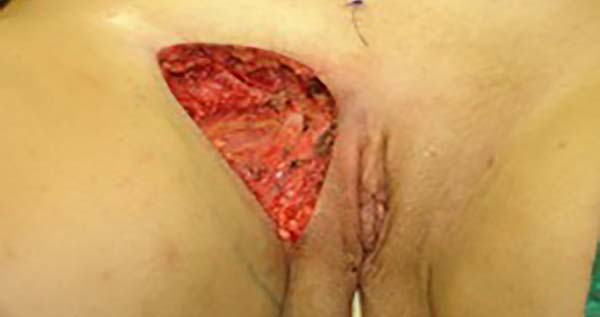
Figure 2 - Defect in the inguinal region and the labia majora after the endometriosis resection.
Figure 2 - Defect in the inguinal region and the labia majora after the endometriosis resection.
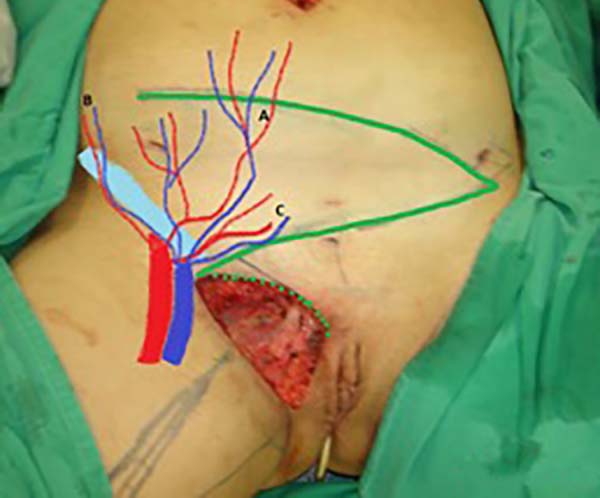
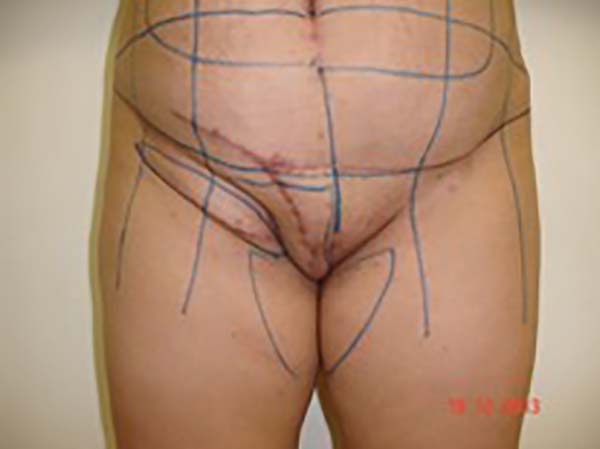
Figure 3 - Measurement of the defect and delineation of a large lower abdominal flap. Diagram
of possible blood supply sources to the flap: inferior superficial epigastric artery
(A), superficial iliac circumflex artery (B), and external pudendal artery (C).
Figure 3 - Measurement of the defect and delineation of a large lower abdominal flap. Diagram
of possible blood supply sources to the flap: inferior superficial epigastric artery
(A), superficial iliac circumflex artery (B), and external pudendal artery (C).
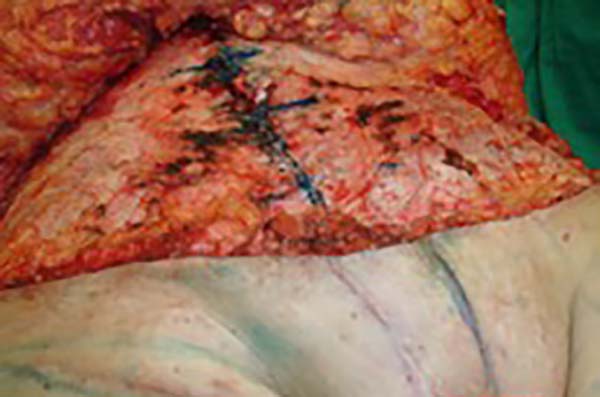
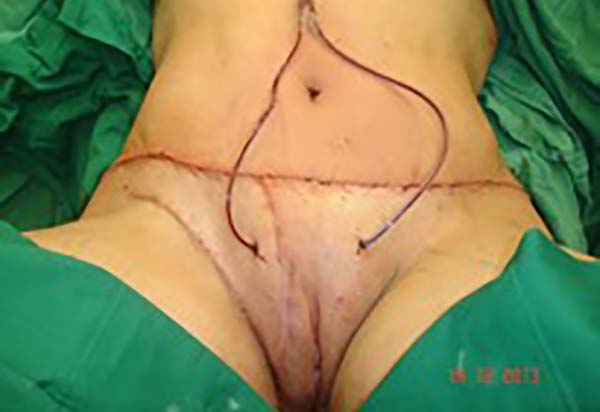
In the first surgical procedure, larger detachments were not made in the umbilical
region to prevent the development of endometriotic implants in other sites; only restoration
of the abdominal wall with plication of the rectus abdominus muscle and neo-omphaloplasty
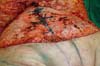
were performed (Figures 4 and 5). In the inguinal region, the defect (approximately 10 cm × 15 cm) was reconstructed
using a laterally based random lower abdominal flap as one of the vertices of a Z-plasty
(Figure 6).
Figure 4 - Abdominal wall defect in the periumbilical region and detached flap.
Figure 4 - Abdominal wall defect in the periumbilical region and detached flap.
Figure 5 - Umbilical stump (new umbilicus) and plication of the rectus abdominus muscle.
Figure 5 - Umbilical stump (new umbilicus) and plication of the rectus abdominus muscle.
Figure 6 - Flap transposition to the inguinogenital region and reconstruction of a new umbilicus.
Figure 6 - Flap transposition to the inguinogenital region and reconstruction of a new umbilicus.
To repair the inguinal canal, Prolene® mesh was attached to the remaining aponeuroses and the pectineal line of the ischium
(Figure 7). Among the possible choices (fasciocutaneous flap of the thigh muscles and adjacent
muscles), we opted for the fasciocutaneous flap of the right iliac fossa, which did
not require further detachment (its design was similar to that of Z-plasty).
Figure 7 - Reconstruction of the inguinal canal defect using Prolene® mesh.
Figure 7 - Reconstruction of the inguinal canal defect using Prolene® mesh.
The surgical team discussed with the patient the possibility of a second approach
involving abdominoplasty at the height of the lesion excision scar, liposculpture
with liposuction of the flap, and symmetrization with fat grafting (Figures 8 and 9).
Figure 8 - Result of the first surgical procedure with adequate tissue volume in the vulvar and
inguinal regions. Planning of the second surgical procedure included lipoabdominoplasty
and flap thinning in the inguinal region.
Figure 8 - Result of the first surgical procedure with adequate tissue volume in the vulvar and
inguinal regions. Planning of the second surgical procedure included lipoabdominoplasty
and flap thinning in the inguinal region.
Figure 9 - Result of the first surgical procedure with adequate tissue volume in the vulvar and
inguinal regions. Planning of the second surgical stage included lipoabdominoplasty
and flap thinning in the inguinal region.
Figure 9 - Result of the first surgical procedure with adequate tissue volume in the vulvar and
inguinal regions. Planning of the second surgical stage included lipoabdominoplasty
and flap thinning in the inguinal region.
After 3 months, the patient’s status had a good evolution without significant complications,
and she underwent a second surgical procedure involving liposculpture abdominoplasty
and flap thinning, i.e., liposuction of the right labia majora and inguinal region
and fat grafting in the left labia majora for symmetrization (Figure 10).
Figure 10 - Result of the second surgical procedure including liposuction and fat grafting in
the vulvar region.
Figure 10 - Result of the second surgical procedure including liposuction and fat grafting in
the vulvar region.
Two years and one month after the second surgical procedure, the patient underwent
a mastopexy with breast augmentation, at which time new procedures (liposuction and
fat grafting) were performed that produced good results (Figures 11 and 12). The endometriosis did not recur, and no significant complications resulted from
the three surgical procedures.
Figure 11 - Result of the second surgical procedure and laser treatment of the scars.
Figure 11 - Result of the second surgical procedure and laser treatment of the scars.
Figure 12 - Late outcome of surgical reconstruction. After the second surgical procedure, further
improvements were made at the time of mastopexy and breast augmentation.
Figure 12 - Late outcome of surgical reconstruction. After the second surgical procedure, further
improvements were made at the time of mastopexy and breast augmentation.
DISCUSSION
Most cases of perineal, vulvar, and abdominal wall endometriosis occur at or near
surgical scars, probably due to mechanical transplantation of endometrial tissue during
episiotomy, vulvar surgery, or accidental trauma9. In contrast, invasion of the lymphovascular space is considered the best explanation
for the pathogenesis of the spontaneous development of perineal lesions9.
Endometriosis is a chronic estrogen-dependent condition that affects 6-10% of women
of reproductive age. Ectopic endometrial tissue can appear anywhere in the body, whereas
endometriotic implants tend to occur in the pelvic region10. Yela et al. (2017)11 observed that endometriotic lesions were located predominantly in previous cesarean
section scars. In contrast, the incidence of abdominal wall endometriosis is low.
The abdominal wall is impaired in 0.03-3.50% of cases of endometriosis, and some studies
reported rates of up to 12%10,11. However, endometriosis has been observed in surgical incisions after conventional
and laparoscopic hysterectomy, appendectomy, and inguinal hernia repair. In these
cases, lesions that were frequently evaluated by a general surgeon for diagnostic
confirmation were misdiagnosed as hernia, hematoma, granuloma, abscess, or lipoma11. Moreover, extrapelvic endometriosis is relatively rare10.
The four diagnostic signs of extrapelvic endometriosis are the intermittent enlargement
of endometriotic lesions, increased sensitivity of lesions near the menstrual period,
dyspareunia, and bleeding9.
Pedunculated skin flaps, including local and regional flaps, are commonly used for
vulvar reconstruction12. Defects (<20 cm2) of the upper third of the vulva can be closed with local flaps such as rhomboid
and V-Y flaps. Partial-thickness skin grafts are an option in patients who do not
require or had no previous radiotherapy. More elaborate flaps may be necessary for
larger defects8,13,14.
Defects of the middle third of the labia majora can be repaired using pudendal-thigh
(Singapore), gracilis myocutaneous, and gluteal myocutaneous flaps8,13.
In the lower third of the vagina or in the vaginal/perianal orifice, defects are best
repaired using gluteal fold flaps, which were initially described by Yii and Niranjan
in 0000x and modified by Hashimoto et al. (1999)8.
Perineal reconstruction is a significant surgical challenge because of the need to
restore urogenital and anorectal functions. This type of procedure impacts multiple
tissues and systems that may become contaminated with bacteria present in flaps and
grafts, increasing the risk of infections and wound dehiscence. The perineum is also
subject to significant pressure from the reclining and sitting positions, potentially
exposing the surgical wound to ischemic pressure and necrosis8,13.
Deep inferior epigastric perforator flaps are commonly used and contain a thick subcutaneous
fat layer12.
Several flaps are available for reconstructing the inguinal region, including those
derived from the sartorius, rectus abdominis, tensor fascia lata, vastus lateralis,
lateral and anterior thigh, and gracilis muscles15.
Sbitany et al. (2010)15 recommended rectus femoris myocutaneous flaps for repairing defects in the inguinal
region. The advantages of these flaps include an arc of rotation sufficient to cover
the entire region and the presence of a reliable vascular pedicle.
In our study, the lower abdominal flap was prepared and achieved good functional and
aesthetic results. Vulvar reconstruction is usually limited to the skin and subcutaneous
tissues and involves restoring the genitalia and producing good aesthetic and functional
results8.
The donor area endured minimal damage that was repaired by abdominoplasty, which provided
a good flap for repairing the defect in the inguinal region and the right labia majora.
During the surgical procedure, polypropylene mesh was placed in the inguinal region
to prevent possible herniation in this region and fat grafting to improve the aesthetic
results.
Many flaps are available for correcting defects in the labia majora and inguinal region,
including V-Y and rhomboid flaps using gracilis, buttock, and thigh muscles. This
study reported an alternative approach for reconstructing a body region for which
surgical repair is a challenge based on the patient’s technical and physiological
difficulties and involved the use of a random fasciocutaneous flap to which the blood
vessels of the lateral abdominal wall provided the vascular supply.
CONCLUSION
The use of a laterally based random lower abdominal flap as one of the vertices of
a Z-plasty is an alternative for reconstructing the inguinal and vulvar regions in
cases of large defects. This technique achieves satisfactory aesthetic results in
these regions without impairing shape or volume while preserving the natural groove
in the thigh root. Furthermore, the thickness of the dermal fat allows functional
remodeling with liposuction and fat grafting.
COLLABORATIONS
|
JDLGA
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Data Curation, Final manuscript approval, Investigation, Methodology, Project Administration,
Supervision, Writing - Original Draft Preparation
|
|
JGOJ
|
Data Curation, Review & Editing
|
|
RSCC
|
Analysis and/or data interpretation, Conception and design study, Data Curation, Realization
of operations and/or trials, Resources, Writing - Original Draft Preparation, Writing
- Review & Editing
|
|
ACC
|
Analysis and/or data interpretation
|
|
RCSD
|
Analysis and/or data interpretation
|
|
AAD
|
Analysis and/or data interpretation
|
|
JCD
|
Analysis and/or data interpretation
|
REFERENCES
1. Acetta I, Acetta P, Acetta AF, et al. Endometrioma de parede abdominal. Arq Bras Cir
Dig. 2011 Mar;24(1):26-29. DOI: https://doi.org/10.1590/S0102-67202011000100006
2. Cervini P, Wu L, Shenker R, et al. Endometriosis in the canal of Nuck: Atypical manifestations
in an unusual location. Can J Plast Surg. 2004;12(2):73-75. DOI: https://doi.org/10.1177/229255030401200202
3. Pontrelli G, Cozzolino M, Stepniewska A, et al. Primary vaginal adenosarcoma with
sarcomatous overgrowth arising in recurrent endometriosis: feasibility of laparoscopic
treatment and review of the literature. J Minim Invasive Gynecol. 2016 Jul/Aug;23(5):833-8.
DOI: https://doi.org/10.1016/j.jmig.2016.03.019
4. Eyvazzadeh AD, Smith YR, Lieberman R, et al. A rare case of vulvar endometriosis in
an adolescent girl. Fertil Steril. 2009 Mar;91(3):929.e9-11. DOI: https://doi.org/10.1016/j.fertnstert.2008.10.026
5. Kondo W, Ribeiro R, Trippia C, Zomer MT. Endometriose profunda infiltrativa: distribuição
anatômica e tratamento cirúrgico. Rev Bras Ginecol Obstet. 2012 Jun;34(6):278-84.
DOI: https://doi.org/10.1590/S0100-72032012000900006
6. Odobasic A, Pasic A, Iljazovic-Latifagic E, et al. Perineal endometriosis: a case
report and review of the literature. Tech Coloproctol. 2010;14(Suppl 1):S25-7. DOI:
https://doi.org/10.1007/s10151-010-0642-8
7. Peloggia A, Petta C. Endometriose profunda: como abordar?. Femina. 2011 Set;39(9):451-457.
8. Weichman KE, Matros E, Disa JJ. Reconstruction of peripelvic oncologic defects. Plast
Reconstr Surg. 2017 Oct;140(4):601e-612e. PMID: 28953736 DOI: https://doi.org/10.1097/PRS.0000000000003703
9. Nasu K, Okamoto M, Nishida M, Narahara H. Endometriosis of the perineum. J Obstet
Gynaecol Res. 2013 May;39(5):1095-7. PMID: 23496239 DOI: https://doi.org/10.1111/jog.12003
10. Okoshi K, Mizumoto M, Kinoshita K. Endometriosis-associated hydrocele of the canal
of Nuck with immunohistochemical confirmation: a case report. J Med Case Rep. 2017;11:354.
DOI: https://doi.org/10.1186/s13256-017-1522-x
11. Yela DA, Trigo L, Bennetti-Pinto CL. Evaluation of cases of abdominal wall endometriosis
at Universidade Estadual de Campinas in a period of 10 years. Rev Bras Ginecol Obstet.
2017 Aug;39(8):403-407. DOI: https://doi.org/10.1055/s-0037-1603965
12. Zeng A, Qiao Q, Zhao R, Song K, Long X. Anterolateral thigh flap-based reconstruction
for oncologic vulvar defects. Plast Reconstr Surg. 2011 May;127(5):1939-45. PMID:
21532422 DOI: https://doi.org/10.1097/PRS.0b013e31820e9223
13. Hollenbeck ST, Toranto JD, Taylor BJ, et al. Perineal and lower extremity reconstruction.
Plast Reconstr Surg. 2011 Nov;128(5):551e-563e. PMID: 22030517 DOI: https://doi.org/10.1097/PRS.0b013e31822b6b87
14. Lee PK, Choi MS, Ahn ST, et al. Gluteal fold V-Y advancement flap for vulvar and vaginal
reconstruction: a new flap. Plast Reconstr Surg. 2006 Aug;118(2):401-6. PMID: 16874210
DOI: https://doi.org/10.1097/01.prs.0000227683.47836.28
15. Sbitany H, Koltz PF, Girotto JA, Veja SJ, Langstein HN. Assessment of donor-site morbidity
following rectus femoris harvest for infrainguinal reconstruction. Plast Reconstr
Surg. 2010 Sep;126(3):933-40. DOI: https://doi.org/10.1097/PRS.0b013e3181e604a1
1. Hospital Daher Lago Sul, Brasília, DF, Brazil.
Corresponding author: Jefferson Di Lamartine Galdino Amaral SCN Quadra 2 Ed. Liberty Mall Torre A, Salas 1121 e 1123, Asa Norte, Brasília, DF,
Brazil. Zip Code: 70297-400. E-mail: jefferson@dilamartine.com.br
Article received: February 10, 2019.
Article accepted: June 10, 2019.
Conflicts of interest: none.