INTRODUCTION
It is well known that the facial nerve can be injured in virtually every face
lift. Despite its importance, it is difficult to find literature on this topic.
Most articles on rhytidoplasty and facial nerve injury are from the early 70’s
to the late 80’s and focus almost exclusively on how to avoid facial nerve
lesions through anatomic dissections. There are very few published studies on
the management of nerve injury and they focus mostly on treatment of complete
nerve transection which comprises 2.6% of operated cases. It is also surprising
that on searching for the terms: “face lift/lifting + facial palsy (FP)” or
“rhytidoplasty/rhytidectomy + facial palsy”, in the PubMed database, there are
no answers that fulfill the search criteria1,2.
Currently there is no concise data regarding the incidence of partial or total
facial nerve injury during cosmetic facial procedures, as it is probably under
reported. A complete or an incomplete facial paralysis after a facial procedure,
may lead to a very uncomfortable situation between the patient and the surgeon,
which is why we recommend a guide in this article to help avoid, identify, and
manage a facial nerve injury in the event of a face lift surgery.
DISCUSSION
Assessment - Facial palsy before surgery: Pre-operative Clinical
Examination.
Despite our best efforts to avoid nerve injury during a face lift surgery, it
may still occur. However, it is important to note that in a significant
number of cases, patients may already have a certain degree of facial
paralysis or pre-operative weakness that remains unnoticed in a routine
consultation. It is quite difficult to identify the signs of a very mild
palsy if the surgeon is not used to treat such cases on a regular basis.
Even patients may not have noticed any degree of asymmetry until the surgeon
points it out but will certainly refer to it in a post- op situation.
To make the pre-op facial assessment as simple as possible, we suggest a
systematic approach to the facial examination. A structured history and
clinical examination of the patient allows for accurate treatment planning
and anticipation of problems that may be exacerbated by surgery3.
Evaluation of facial asymmetry and spontaneity of facial motion can be
performed while eliciting a routine medical history. With regard to
functional history, a “top down” approach is utilized in a systematic
manner, beginning with the brow. An ophthalmic and nasal history is
obtained, and the patient is questioned regarding oral continence and
speech. The surgeon should always include a history of Bell’s palsy.
Recurrence or new onset palsy, three to five weeks post-op in our experience
is possible but rare and undesirable.
A physical examination is also performed from the brow downwards. The
presence or absence of rhytids and brow ptosis is noted. An asymmetric brow
ptosis is common and is seen clearly over a period of time in photos. The
upper lid is also examined for dermatochalasis and lid retraction. The
patient is asked to close the eyes and any lagophthalmos is measured. The
lower lid position is inspected for an ectropion and a snap test is
performed3.
The nose is examined to exclude any fixed nasal obstruction and a Cottle’s
test is performed to determine any nasal valve collapse4. Any midface ptosis or nasolabial crease asymmetry is
evaluated. The mouth is examined at rest, with the amount of commissure
droop and deviation of the philtrum to the contralateral side measured, if
present. The excursion of the commissure is quantified and the degree of
tooth show and shape of the smile are noted5-7. The lower
lip is observed for any signs of weak depressor anguli oris function,
indicating involvement of the mandibular division8. It is also very important to take sequential photos
of the patient, at rest and in motion, before and after surgery, and note if
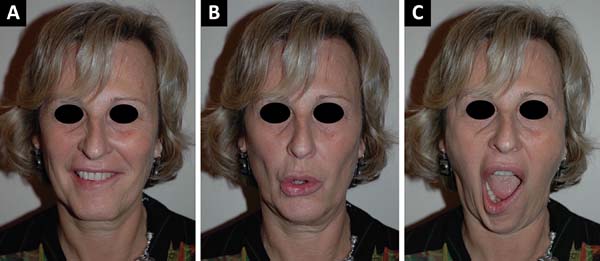
there are any complications (Figura 1).
Figure 1 - Pre-operative clinical examination showing minimal facial
palsy: mandibular branch. A: Slightly visible
during smiling; B: Protruding the lips;
C: More visible while opening the mouth
(Personal archives).
Figure 1 - Pre-operative clinical examination showing minimal facial
palsy: mandibular branch. A: Slightly visible
during smiling; B: Protruding the lips;
C: More visible while opening the mouth
(Personal archives).
Avoiding facial nerve injury during a face lift
Several published articles have focused on this particular topic. It is clear
that a deep knowledge of the facial anatomical structures and the anatomy of
the facial nerve is very important. We recommend an article published in the
late 70’s by Baker2 and a textbook by
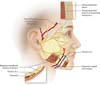
Brook Seckel titled: Facial Danger Zones (Figura 2)9, for surgeons
who perform face lifts. Nevertheless, to master facial anatomy, we should
always dissect in a very meticulous way, because the anatomy of high-risk
areas is extremely variable and changes from patient to patient2,10.
Figure 2 - Facial “danger zones” according to Seckel, encompassing the
temporal and marginal mandibular branches of the facial nerve,
respectively
9.
Figure 2 - Facial “danger zones” according to Seckel, encompassing the
temporal and marginal mandibular branches of the facial nerve,
respectively
9.
Attention to minute details is as important as the dissection itself. Caution
should be used while injecting solutions using fine needles or small
cannulas. We are very permissive with the volume injected, using about 200cc
of a 1:500.000 adrenaline solution. Intra-op anesthetic solutions are best
avoided. Hydro dissection is recommended as it facilitates the undermining
tissue and avoids unnecessary sharp dissections which could lead to less
risk of inadvertent lesions and hematoma formation.
A blunt dissection is preferred especially for sub Superficial muscular
aponeurotic system (SMAS) techniques. Trepsat dissectors (Pouret medical)
are used routinely for both facial and neck approaches which makes a
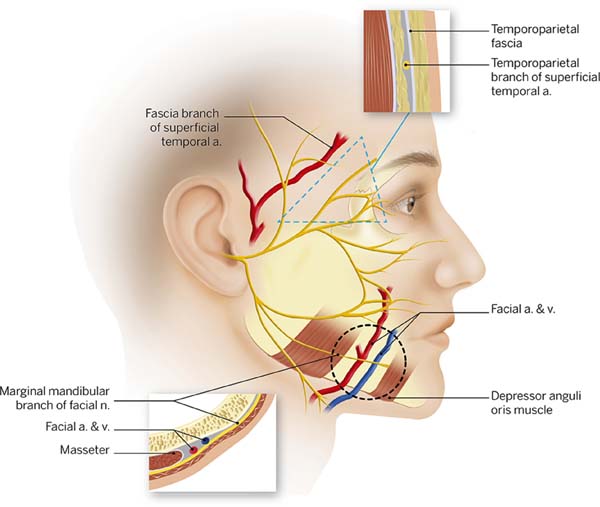
complete nerve transection virtually impossible (Figura 3).
Figure 3 - Picture according to May, representing common areas of nerve
injury during a facelift and the associated anatomical
features
30.
Figure 3 - Picture according to May, representing common areas of nerve
injury during a facelift and the associated anatomical
features
30.
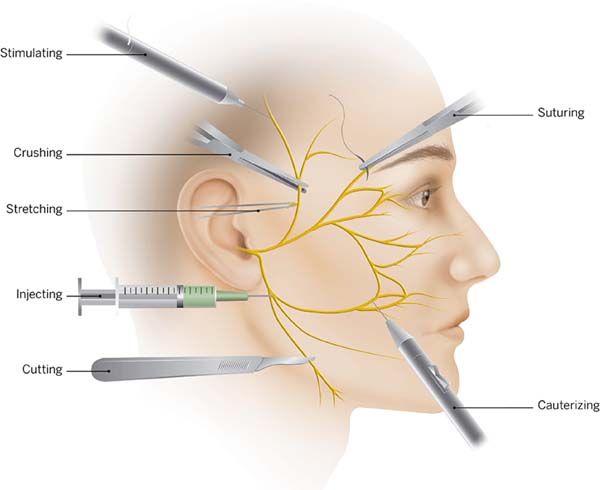
Suturing and hemostasis techniques are also important. Avoid placing deep
sutures in the SMAS, and preferentially place them along the axis of the
major facial nerve branches. A surgeon must also prevent excessive traction
and undue stretching2. During
hemostasis, use of a bipolar cautery is advised and not large clamps or
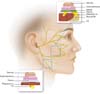
forceps, to minimize the electrocoagulation trauma (Figura 4). If there is a doubt regarding a nerve branch
injury, a neurostimulator must be readily available at the operation room,
to confirm any suspicion. It is important that the patient should not be
curarized.
Figure 4 - Schema showing tips on how to avoid facial nerve injury
during a facelift dissection, according to Baker
2.
Figure 4 - Schema showing tips on how to avoid facial nerve injury
during a facelift dissection, according to Baker
2.
Nerve Section observed per-op
Occasionally the surgeon will be able to identify the damaged branch of the
facial nerve. As the dissection goes towards the nasolabial fold, the nerves
become less thick, which makes it difficult to correctly identify the
anatomy with the naked eye. Several studies have been published describing
surface anatomy landmarks correlating to the nerve divisions12-14 but the logical step is to test the assumed damaged
branch and the surrounding ones with a neurostimulator, to avoid
misidentification.
After this step, the nerve is sutured. Dissection of the proximal and distal
ends of the nerve is done under magnification. A check is done to see if
there is any loss of substance and an epineural suture is performed with
non-absorbable 10 or 9.0 nylon. In case of traction of the nerve, nerve
grafts are recommended, which are usually harvested from the great auricular
nerve or the sural nerve15.
Post op paralysis: What should be done?
Diagnostic
In a majority of cases the surgeon will face the onset of paralysis
during post-op. Whenever possible, evaluating the facial mimetic muscles
at the end of the surgery is a good way to differentiate surgical trauma
from other post-op causes such as Bell’s palsy. It is important to have
a very strict follow-up and take pictures at every single contact with
the patient.
To clinically assess the severity of peripheral facial nerve palsy
various scoring systems are available. In our opinion, the most suitable
facial nerve grading system is the Muscle testing of Freyss (Chart 1). This scale allows each
muscle function to be evaluated separately, which is different from
other grade systems, such as the House-Brackmann Grading System, which
assesses groups of facial muscles. The system relies on evaluation of
the degree of voluntary excursion of the facial muscles, evaluating ten
muscles groups and attributing scores from 0 to 3 for each muscle, for a
total score ranging from 0 to 3016. Evaluation is usually limited to two or three muscles,
according to the injured nerve branch.
Chart 1 - Muscle testing of Freyss
16.
| Ten facial muscles |
Score |
Muscular contraction |
| Frontalis |
Wrinkles forehead and raises
eyebrows
|
0 |
No contraction |
| Corrugator Supercilii |
Pulls eyebrows medially and down |
1 |
Minimal contraction |
| Orbicularis oculi |
Closes eyelids |
2 |
Wide excursion but weak contraction |
| Procerus |
Pulls medial angle of eyebrow down
producing wrinkles over bridge of nose
|
3 |
Normal contraction |
| Dilator naris muscle |
Expands the nostrils |
| Orbicularis oris |
Closes and protrudes lips |
Total Score (0-30) |
Grade of Facial palsy |
| Risorius |
Pulls corner of mouth lateral |
Score 20-30 |
Slight |
| Zygomaticus major |
Pulls corner of mouth up and lateral |
Score 10-20 |
Mild |
| Buccinator |
Compresses cheek against teeth |
Score 0-10 |
Severe |
| Mentalis |
Depresses lower lip and wrinkles chin
skin
|
Score 0 |
Total |
Chart 1 - Muscle testing of Freyss
16.
Facial nerve palsy can be categorized as complete if there is inability
to voluntarily contract the facial muscles, or incomplete (partial). The
degree of nerve damage can also be assessed by nerve conduction studies
(electromiography-EMG) of the facial nerve. Reduction of the compound
muscle action potential suggests axonal degeneration whereas increase in
latency suggests demyelination of the nerve17.
Facial nerve palsy is an extremely frightening situation for the patient.
The most frequently asked questions by these patients are whether their
facial function will return to normal and how long it will take.
Among the wide variety of available prognostic tests, as discussed by
Hughes18, the EMG seems to be
the most reliable test to predict a patient’s prognosis19. To evaluate the lesion, an EMG
is done early in the post-op, between day 4 and 6, although there is no
rule ordering this first exam, because at this early stage,
electromyography is used to detect any remaining voluntary activity. If
voluntary potentials are detected, the palsy is labeled incomplete. The
paralysis is considered complete only in the case of a totally silent
electromyography19.
A diagnosis according to Sunderland and Seddon classifications (Chart 2) cannot be made at this
stage, because pathologic spontaneous activity as a sign of neural
degeneration does not occur earlier than 10 to 14 days after the onset
of palsy. This early test is mainly intended to categorize the severity
of an individual palsy and does not yield reliable prognostic
information20,21.
Chart 2 - Sunderland and Seddon classifications
21.
| Sunderland |
Seddon |
Injury |
Recovery potential |
| I |
Neurapraxia I |
Disruption of the nerve conduction, but
nerve structure and axon remain intact.
|
Full (Up to 12 weeks) |
| II |
Axonotmesis II |
Disruption of the nerve conduction and
axon degeneration, but remaining nerve structure remains
intact.
|
Full (1mm/day) |
| III |
Axonotmesis II |
Disruption of the endoneurium, but the
perineurium and epineurium remains intact.
|
Full (1mm/day) |
| IV |
Axonotmesis II |
Disruption of the endoneurium and
perineurium, but epineurium remains intact.
|
Poor to none |
| V |
Neurotmesis III |
Total transection of the nerve
fiber.
|
None |
Chart 2 - Sunderland and Seddon classifications
21.
A second examination should be performed not earlier than 10 to 14 days
after the onset of palsy. After this period, all diagnostic criteria may
have developed to establish a diagnosis according to Seddon, which
predicts a prognosis on the expected clinical course19,20.
Steinner published that a fibrillation detected in EMG studies later than
10 to 14 days predicts that a patient has an 80% chance of an
unfavorable result, but on the other hand, the absence of these signs
implies an approximately 93% chance of total recovery20.
Treatment
Eye protection
One of the biggest problems with upper face facial palsy is the
involvement of the eye if the lid commissure remains open. In this
situation, eye care focuses on protection of the cornea from
dehydration, drying or abrasions due to insufficient lid closure or
tears. Eye ointments are recommended during day and night with
protective glasses during the day22.
Physiotherapy
There are only a few controlled trials available on the effectiveness of
physical therapy for facial palsies. In a randomized trial on 50
patients with Bell’s palsy and a House Brackmann scale grade of IV, mime
therapy, speech therapy, including automassage, relaxation exercises,
inhibition of synkinesis, coordination exercises, or emotional
expression exercises, resulted in improvement of facial stiffness, lip
motility, and the physical and social indices of the facial disability
index23. A simple and
reproducible technique has been used and advised by us: the mirror
feedback therapy. It involves training the paralyzed side to reproduce
symmetrical movements of the unaffected side in front of a mirror.
Blanchin et al.24, in 2013,
presented a paper proving that when the mirror therapy is applied to
patients with long standing facial palsy and submitted to Labbe’s
technique of facial reanimation it is more effective in recovering a
spontaneous smile when compared to conventional therapies.
Corticosteroids
Till date to our knowledge, no study has discussed facial nerve trauma
and the use of steroids for treatment. But since it is known that
inflammation (particularly edema) of the facial nerve plays a key role
in the pathogenesis of other types of facial paralysis, such as Bell’s
palsy, we can extrapolate the concept to surgical trauma,. given the
fact that a vast majority of cases are partial lesions.
Corticosteroids have been used due to its powerful anti-inflammatory
effects in Bell’s palsy and this has recently been supported by a
growing and well-designed evidence base. A Cochrane review included 1569
patients from 8 randomized controlled trials of adequate quality, and
showed a benefit in improving the facial recovery, and a significant
reduction in motor synkinesis in the steroid group25. Another high level systematic review published
in the Journal of the American Medical Association (JAMA) concluded that
corticosteroids used alone produced a reduced risk of unsatisfactory
recovery26.
Even though the reviews support the use of steroids in facial palsy,
there is no consensus on the prescription pattern. There are many
authors who have suggested different protocols. We recommend the ones
with the largest reviews:
- The Sullivan protocol27:
Prednisolone 25 mg, by mouth (PO), twice daily, for 10 days, starting at
a maximum of 72 h from the onset of palsy.
- The Engström protocol28:
Prednisolone 60 mg PO for 5 days, then the dose is reduced by 10 mg per
day for 5 days, also starting before 72 h of the onset.
- The Lagalla Protocol29:
Prednisone 1 g, intravenous (IV), for 3 days, than 0.5 g IV for 3
days.
- The Stennert protocol (Table 1)30.
Table 1 - Stennert protocol
30.
| Days of
treatment
|
Cortisone (Prednisolone -
equivalent dose - mg/day)
|
Dextran 40 with sorbite or
mannite 5-10%c (ml)
|
Pentoxifylline (Trental)
(ml)
|
| |
<70kg |
|
>70kg |
| In-patient |
1 |
Infusiona |
200 |
|
250 |
500 |
5 |
| |
2 |
|
200 |
|
250 |
500 |
10 |
| |
3 |
|
|
150 |
|
500 |
15 |
| |
4 |
|
|
150 |
|
500 |
15 |
| |
5 |
|
|
100 |
|
500 |
15 |
| |
6 |
|
|
100 |
|
500 |
15 |
| |
7 |
|
|
75 |
|
500 |
15 |
| |
8 |
|
|
50 |
|
500 |
15 |
| |
9 |
Oral
circadianb |
40 |
|
500 |
15 |
| |
10 |
20 |
|
500 |
15 |
| Out-patient |
11 |
(6-8 a.m.) |
15 |
|
|
|
|
| |
12 |
|
12.5 |
|
|
|
|
| |
13 |
|
10 |
|
|
|
|
| |
14 |
|
7.5 |
|
|
|
|
| |
15 |
|
5 |
|
|
|
|
| |
16 |
|
2.5 |
|
|
|
|
| |
17 |
|
2.5 |
|
|
|
|
| |
18 |
|
2.5 |
|
|
|
|
Table 1 - Stennert protocol
30.
Stennert30 proposed a protocol
based on an assumption that nerve damage is caused by edema and primary
and secondary ischemia. To reduce the phlogistic and edematous reaction,
he introduced steroids. Secondly he tried to increase the peripheral
nerve perfusion, by adding pentoxifyline and dextrane to the IV
infusion.
The effect of pentoxifylline on the recovery of Bell’s palsy has only
been tested in association with other drugs, particularly steroids and
low-molecular dextran. The studies showed a beneficial effect of a
combination therapy, but it is not known which of these drugs is
responsible for the beneficial effect16,28.
Botulinum toxin
When injected into facial muscles, botulinum toxin has been found to
reduce the facial asymmetry in patients suffering from facial paralysis
and has being used to treat synkinesis, hyperlacrimation, and
hyperkinesis9. Most people
have neglected the “physiotherapeutic” effect of the toxin. When applied
to the healthy side, the toxin moderates movements, forcing the patient
to exercise the affected side, which helps the muscle recover, and
stimulates new neural connections. Therefore we recommend that it should
be utilized even in the absence of hyperkinesia after confirmation of
the diagnosis, around 12 days after the surgery (assumed facial palsy
onset) and the EMG.
CONCLUSION
There is no consensus on management for a case of accidental nerve injury,
therefore we have proposed a protocol with safe technical options to avoid nerve
damage, identify, and treat if necessary. The proposed protocol is based on our
own experience on treating facial palsy and published studies . Although it
seems difficult to deal with such cases in general, patients with partial nerve
lesions have an excellent prognosis with a recovery rate of 90 to 94%20, especially when the right decisions are
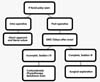
made at the right time. In summary, we present a flowchart to help make clinical
decisions (Figura 5).
Figure 5 - Decision flowchart: Clinical decision making in facial nerve
injuries.
Figure 5 - Decision flowchart: Clinical decision making in facial nerve
injuries.
COLLABORATIONS
|
FSR
|
Analysis and/or data interpretation, conception and design study,
data curation, final manuscript approval, methodology, project
administration, supervision, visualization, writing - original draft
preparation, writing - review & editing.
|
|
CMR
|
Data curation, supervision, visualization, writing - review &
editing.
|
|
FV
|
Supervision, writing - review & editing.
|
|
DL
|
Analysis and/or data interpretation, conception and design study,
data curation, final manuscript approval, methodology, project
administration, supervision.
|
REFERENCES
1. Castañares S. Facial nerve paralyses coincident with, or subsequent
to, Rhytidectomy. Plast Reconstr Surg. 1974;54(6):637-43.
2. Baker DC, Conley J. Avoiding facial nerve injuries in rhytidectomy.
Anatomical variations and pitfalls. Plast Reconstr Surg.
1979;64(6):781-95.
3. Fattah A, Borschel GH, Manktelow RT, Bezuhly M, Zuker RM. Facial
Palsy and Reconstruction. Plast Reconstr Surg.
2012;129(2):340e-52e.
4. Howard BK, Rohrich RJ. Understanding the nasal airway: principles
and practice. Plast Reconstr Surg. 2002;109(3):1128-46.
5. Manktelow RT, Zuker RM, Tomat LR. Facial paralysis measurement with
a handheld ruler. Plast Reconstr Surg. 2008;121(2):435-42.
6. Paletz JL, Manktelow RT, Chaban R. The shape of a normal smile:
implications for facial paralysis reconstruction. Plast Reconstr Surg.
1994;93(4):784-9.
7. Rubin LR. The anatomy of a smile: its importance in the treatment of
facial paralysis. Plast Reconstr Surg. 1974;53(4):384-7.
8. de Maio M, Bento RF. Botulinum toxin in facial palsy: an effective
treatment for contralateral hyperkinesis. Plast Reconstr Surg.
2007;120(4):917-27.
9. Seckel BR. Facial danger zones: avoiding nerve injury in facial
plastic surgery. 1a ed. St Louis: Quality Medical Publishing;
1994.
10. Roostaeian J, Rohrich RJ, Stuzin JM. Anatomical considerations to
prevent facial nerve injury. Plast Reconstr Surg.
2015;135(5):1318-27.
11. May M, Schaitkin BM. The facial nerve. New York: Thieme;
2000.
12. Chatellier A, Labbé D, Salamé E, Bénateau H. Skin reference point
for the zygomatic branch of the facial nerve innervating the orbicularis oculi
muscle (anatomical study). Surg Radiol Anat. 2013;35(3):259-62.
13. Stuzin JM, Wagstrom L, Kawamoto HK, Wolfe SA. Anatomy of the frontal
branch of the facial nerve: the significance of the temporal fat pad. Plast
Reconstr Surg. 1989;83(2):265-71.
14. Dorafshar AH, Borsuk DE, Bojovic B, Brown EN, Manktelow RT, Zuker
RM, et al. Surface anatomy of the middle division of the facial nerve: Zuker’s
point. Plast Reconstr Surg. 2013;131(2):253-7.
15. Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin
glue for peripheral nerve repair. Plast Reconstr Surg.
2011;127(6):2381-90.
16. Dubreuil C, Charachon R. Clinique: la paralysie faciale
peripherique. In: Charachon R, Bebear JP, Sterkers O, Magnan J, Soudant J, eds.
La paralysie faciale. Le spasme hemifacial. Paris: Société Française
D’oto-Rhino-Laryngologie et de Pathologie Cervico-Faciale / L’européenne
D’éditions; 1997. p. 135-57.
17. Finsterer J. Management of peripheral facial nerve palsy. Eur Arch
Otorhinolaryngol. 2008;265(7):743-52.
18. Hughes GB. Prognostic tests in acute facial palsy. Am J Otol.
1989;10(4):304-11.
19. Grosheva M, Wittekindt C, Guntinas-Lichius O. Prognostic value of
electroneurography and electromyography in facial palsy. Laryngoscope.
2008;118(3):394-7.
20. Sittel C, Stennert E. Prognostic value of electromyography in acute
peripheral facial nerve palsy. Otol Neurotol. 2001;22(1):100-4.
21. Chhabra A, Ahlawat S, Belzberg A, Andreseik G. Peripheral nerve
injury grading simplified on MR neurography: As referenced to Seddon and
Sunderland classifications. Indian J Radiol Imaging.
2014;24(3):217-24.
22. Holland NJ, Weiner GM. Recent developments in Bell’s palsy. BMJ.
2004;329(7465):553-7.
23. Beurskens CH, Heymans PG. Positive effects of mime therapy on
sequelae of facial paralysis: stiffness, lip mobility, and social and physical
aspects of facial disability. Otol Neurotol. 2003;24(4):677-81.
24. Blanchin T, Martin F, Labbe D. Rééducation des paralysies faciales
après myoplastie d’allongement du muscle temporal. Intérêt du protocole «
effet-miroir ». Ann Chir Plast Esthet. 2013;58(6):632-7.
25. Salinas RA, Alvarez G, Daly F, Ferreira J. Corticosteroids for
Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev.
2010;(3):CD001942. DOI: 10.1002/14651858.CD001942.pub4
26. de Almeida JR, Al Khabori M, Guyatt GH, Witterick IJ, Lin VY,
Nedzelski JM, et al. Combined corticosteroid and antiviral treatment for Bell
palsy: a systematic review and meta-analysis. JAMA.
2009;302(9):985-93.
27. Sullivan FM, Swan IR, Donnan PT, Morrison JM, Smith BH, McKinstry B,
et al. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J
Med. 2007;357(16):1598-607.
28. Engström M, Berg T, Stjernquist-Desatnik A, Axelsson S, Pitkäranta
A, Hultcrantz M, et al. Prednisolone and valaciclovir in Bell’s palsy: a
randomised, double-blind, placebo-controlled, multicentre trial. Lancet Neurol.
2008;7(11):993-1000.
29. Lagalla G, Logullo F, Di Bella P, Provinciali L, Ceravolo MG.
Influence of early high-dose steroid treatment on Bell’s palsy evolution. Neurol
Sci. 2002;23(3):107-12.
30. Stennert E. New concepts in the treatment of Bell’s palsy. In:
Malcolm D, House G, House WF, eds. Disorders of the facial nerve. New York:
Raven Press; 1981. p. 313-8.
1. Clínica Cirurgia Plástica Beauté, Belém, PA,
Brazil.
2. Universidade Estadual de Botucatu, Botucatu,
SP, Brazil.
3. Clínica Particular, Caen, Normandia,
França.
Corresponding author: Franklin de Souza Rocha,
Travessa Dom Romualdo de Seixas 1560, Belém, Brazil. Zip Code: 66055-028.
E-mail: franklinrocha1@hotmail.com
Article received: March 02, 2018.
Article accepted: April 16, 2019.
Conflicts of interest: none.