INTRODUCTION
Approximately 1 million people in Brazil suffer burns every year, particularly
second-degree superficial and/or deep burns1,2. The ideal
dressing for such burns is easy to obtain, has good flexibility and adhesion to
the wound bed, resists stretching, is easily handled, can suppress pain, if of
low cost, is simple to store and, above all, prevents hydroelectrolytic losses
and bacterial contamination, promotes epithelization, and encourages the
adequate formation of granulation tissue in cases of grafting3.
Temporary skin substitutes and synthetic/biosynthetic dressing have been
considered useful in the treatment of superficial burns because they reduce the
frequency of dressing changes4. However,
these materials are expensive and ineffective for deep burns5. Thus, alternative biological materials
have been sought for this purpose. Tissues of animal origin, such as porcine
skin and porcine intestinal submucosa, are among the materials used6. Recent studies have suggested the use of
Nile tilapia skin (Oreochromis niloticus) as a biomaterial in
regenerative medicine since it presented good adhesion to the wound bed in
rats3 and satisfactory results of
histological, histochemical, and tissue traction tests with human skin6.
Tilapia skin displays good tensile and compression resistance7, indicating that it may be usable as a
biological dressing for burns. The presence of peptides with possible
antimicrobial functions within this tissue reinforces this possibility 8-10.
OBJECTIVE
This study aimed to evaluate the efficacy of the use of Nile tilapia skin as an
occlusive biological dressing compared to silver-based hydrofiber dressing
(Aquacel AG®) in the management and treatment of superficial
and deep second-degree burns in adults.
METHODS
This analytical interventional open clinical study with a convenience sample was
performed at Hospital São Marcos, Recife/PE. The study was approved by the
Research Ethics Committee of the Federal University of Pernambuco (no.
2.735.537). A clinical evaluation verified the general health conditions and the
inclusion criteriA: Presence of superficial and/or deep
second-degree burns affecting up to 10% of the burned body surface; maximum 72
hours since the burn occurred; age 20–60 years; and the absence of previous
treatment for the current burns or significant comorbidities.
A total of 30 patients were selected. After receiving the initial explanation and
providing written informed consent, they were randomly distributed into two
groups: occlusive biological dressing with Nile tilapia skin (n = 15) or
conventional treatment with the Aquacel AG® silver-based
hydrofiber dressing (n = 15). The therapeutic process is described in Chart 1.
Chart 1 - Therapeutic process applied to patients.
| Visit 1 (screening): |
|
| |
• Collection of written informed
consent
|
| |
• Clinical evaluation - physical
examination, vital signs, anthropometric data;
|
| |
• Evaluation of the eligibility criteria
(inclusion and exclusion criteria);
|
| |
• Allocation to the test or control group
(according to randomization);
|
| |
• Photographs of the wound; |
| |
• Preparation of the dressing; |
| |
Guidelines on the procedures of the protocol and
application of the visual analog scale (VAS) for pain
|
| Treatment visits: |
| |
• Clinical evaluation; |
| |
• Dressing evaluation - verification if
dressing replacement is necessary in the test and control
groups;
|
| |
• Photographs of the wound; |
| |
• Application of the VAS |
| Follow-up Visit - 7 (± 3) days
after withdrawal of the dressing:
|
| |
• Clinical evaluation; |
| |
• Photographs of the wound; |
| |
• Study discharge |
Chart 1 - Therapeutic process applied to patients.
Nile tilapia skins are decontaminated (2% chlorhexidine and glycerol at high
concentrations) and sterilized with gamma irradiation (Cobalt 60) to ensure the
safety of their use in humans in addition to sampling microbiological testing
for Gram-positive and -negative bacteria and fungi (Figure 1).
Figure 1 - Nile tilapia skin sterilized and packaged for human use.
Figure 1 - Nile tilapia skin sterilized and packaged for human use.
The procedures for both groups are described in Chart 2.
Chart 2 - Treatment procedures in the test and control groups.
| |
Procedures |
| First dressing |
• Removal of blisters or loose skin |
| • Washing the lesion with running water and 2%
chlorhexidine
|
| • Application of dressing |
| • Test group: Occlusive biological dressing with Nile
Tilapia skin (n = 15)
|
| • Control group: Conventional treatment with silver
hydrogel (Aquacel AG®) (n = 15)
|
| • Coverage with cotton gauze, crepe bandages, and elastic
tubular netting.
|
| Return |
• Removal of the dressing and gauze
layer
|
| • Evaluation of the dressing for adherence to the wound
bed
|
| • Replacement only when not adhered |
Chart 2 - Treatment procedures in the test and control groups.
The outcomes for this study were:
Number of days required to achieve complete wound healing. The wound
was considered healed when 95% or more of the initial burn area was
re-epithelialized.
Pain assessment using a visual analog scale (VAS). ZERO corresponded
to no pain, while TEN indicated the worst pain felt during cleaning
and after application of the dressing. At each patient visit, the
dressing’s condition was evaluated and the pain score was
recorded.
Number of times a replacement Nile tilapia skin or Aquacel
AG® dressing was required.
The results were analyzed using descriptive statistics of absolute and relative
frequencies and mean and standard deviation. The treatments were evaluated using
Fisher’s exact test with a significance of p < 0.05 using
SPSS version 20.0 software.
RESULTS
Of our cohort, 53.3% were treated with Nile tilapia skin, while 46.6% were
treated with Aquacel AG®.
Table 1 shows that the mean treatment
times in days were similar between the Nile tilapia skin and the Aquacel
AG® (9.6 ± 2.4 and 10.7 ± 4.5 days, respectively).
Table 1 - Descriptive statistics of the number of days (complete epithelization
of the wound) according treatment type applied to second-degree burns in
adults, Hospital São Marcos, Recife/PE - 2018.
| |
Categories |
Treatment type |
P value
|
| |
Nile tilapia skin |
Aquacel AG® |
| Number of days (discharge) |
Minimum |
5 |
4 |
0.36 |
| Maximum |
14 |
19 |
| Average |
9.6 |
10.7 |
| Standard deviation |
2.4 |
4.5 |
Table 1 - Descriptive statistics of the number of days (complete epithelization
of the wound) according treatment type applied to second-degree burns in
adults, Hospital São Marcos, Recife/PE - 2018.
Table 2 shows that the patients in both
groups reported a VAS score greater than 5 during the exchange of dressings
(p > 0.05; Fisher’s exact test).
Table 2 - Statistical value of the pain VAS score during dressing exchange
according to treatment type applied to seconddegree burns in adults,
Hospital São Marcos, Recife/PE - 2018.
| |
|
Treatment type |
Total |
P value
|
| |
|
|
Nile tilapia skin |
Aquacel AG® |
| Pain (during dressing exchange) |
≤ 5 points |
n |
5 |
3 |
8 |
0.68 |
| |
% |
33.3% |
20.0% |
26.7% |
| > 5 points |
n |
10 |
12 |
22 |
| |
% |
66.7% |
80.0% |
73.3% |
| Total |
|
n |
15 |
15 |
30 |
| |
% |
100.0% |
100.0% |
100.0% |
Table 2 - Statistical value of the pain VAS score during dressing exchange
according to treatment type applied to seconddegree burns in adults,
Hospital São Marcos, Recife/PE - 2018.
After the dressing was changed, a new VAS pain score was collected. Table 3 shows that 86.7% of patients
treated with Nile tilapia skin showed a reduced VAS score, and an analysis using
Fisher’s exact test showed that it was not inferior to the Aquacel
AG®.
Table 3 - Statistical value of the pain VAS score after the application of
dressings according to treatment type for seconddegree burns in adults,
Hospital São Marcos, Recife/PE - 2018.
| |
|
Treatment type |
Total |
P value
|
| |
|
|
Nile tilapia skin |
Aquacel AG® |
| Pain (after dressing application) |
≤ 5 points |
n |
13 |
7 |
20 |
≤0.050 |
| |
% |
86.7% |
46.7% |
66.7% |
| > 5 points |
n |
2 |
8 |
10 |
| |
% |
13.3% |
53.3% |
33.3% |
| Total |
|
n |
15 |
15 |
30 |
| |
% |
100.0% |
100.0% |
100.0% |
Table 3 - Statistical value of the pain VAS score after the application of
dressings according to treatment type for seconddegree burns in adults,
Hospital São Marcos, Recife/PE - 2018.
Table 4 presents values regarding the
number of skin substitutions or dressings required for complete
re-epithelialization represented by patient discharge. Note that 60% of the
patients who were treated with the Nile tilapia skin did not require skin
replacement at any time during treatment, whereas 53.3% of patients treated with
Aquacel AG® required more than one dressing replacement
(p = 0.71), which indicates that the Nile tilapia skin was
not inferior to the Aquacel AG®.
Table 4 - Comparison of treatments according to number of dressing exchanges in
the treatment of second-degree burns in adults, Hospital São Marcos,
Recife/PE - 2018.
| |
|
Treatment type |
Total |
P value
|
| |
|
|
Nile tilapia skin |
Aquacel AG® |
| Number of exchanges |
0 |
n |
9 |
7 |
16 |
0.71 |
| |
% |
60% |
46.7% |
53.33% |
| ≥ 1 |
n |
6 |
8 |
14 |
| |
% |
40% |
53.3% |
46.67% |
| Total |
|
n |
15 |
15 |
30 |
| |
% |
100% |
100.0% |
100% |
Table 4 - Comparison of treatments according to number of dressing exchanges in
the treatment of second-degree burns in adults, Hospital São Marcos,
Recife/PE - 2018.
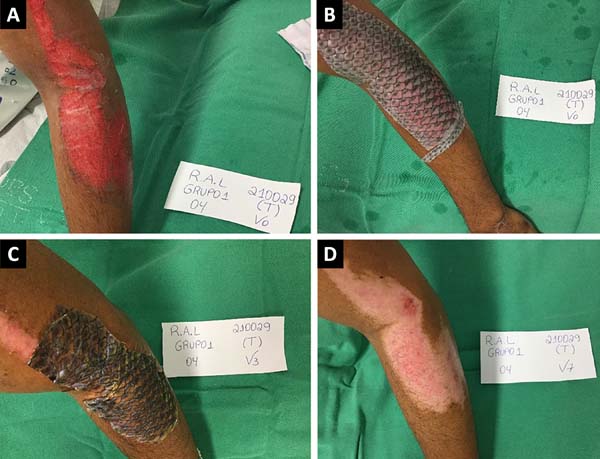
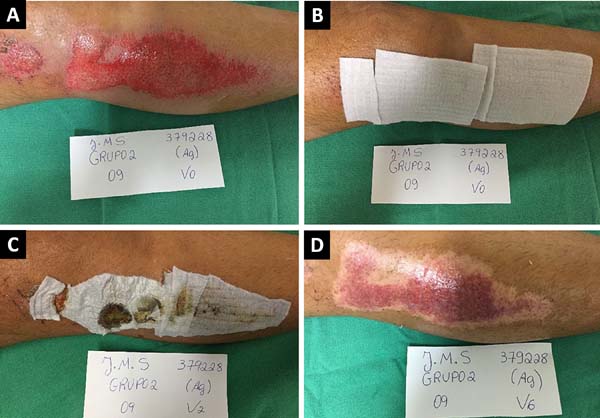
Figures 2 and 3 show the clinical results of two patients in the study from the
first visit until medical discharge (complete re-epithelization).
Figure 2 - Clinical case of a patient treated with occlusive biological
dressing (Nile tilapia skin). A: Wound assessment and
cleaning and visual analog scale (VAS) pain assessment;
B: Dressing with the Nile tilapia skin at the first
clinical appointment and VAS pain assessment; C:
Evaluation of the bandage after 7 days; D: Complete
epithelization of the wound after 16 days.
Figure 2 - Clinical case of a patient treated with occlusive biological
dressing (Nile tilapia skin). A: Wound assessment and
cleaning and visual analog scale (VAS) pain assessment;
B: Dressing with the Nile tilapia skin at the first
clinical appointment and VAS pain assessment; C:
Evaluation of the bandage after 7 days; D: Complete
epithelization of the wound after 16 days.
Figure 3 - Clinical case of a patient treated with a silver-based hydrofiber
dressing (Aquacel AG®). A: Wound assessment and
cleaning and visual analog scale (VAS) pain assessment;
B: Dressing with Aquacel AG® at the first
clinical appointment and VAS pain assessment; C:
Evaluation of the dressing after 7 days; D: Complete
epithelization of the wound after 18 days.
Figure 3 - Clinical case of a patient treated with a silver-based hydrofiber
dressing (Aquacel AG®). A: Wound assessment and
cleaning and visual analog scale (VAS) pain assessment;
B: Dressing with Aquacel AG® at the first
clinical appointment and VAS pain assessment; C:
Evaluation of the dressing after 7 days; D: Complete
epithelization of the wound after 18 days.
DISCUSSION
Studies have shown that hot liquids are the most common thermal agents that cause
burn injuries1,2,11,12. In this
study, 45% of the cases were due to overheated liquids.
The treatment and care of burns aim to provide a suitable environment for
re-epithelialization and control the proliferation of microorganisms, which may
delay the healing process13. Thus,
biological dressings must display properties that prevent microbial growth,
promote epithelization, and encourage the formation of granulation tissue6,14.
Records of the use of silver-based dressings date back to the 18th
century15. Various properties of this
material have been studied, including accelerating healing time, antimicrobial
activity, and rapid re-epithelization. Despite its large-scale use, some
disadvantages, including cytotoxicity, have inspired the study of other
materials12,15,16.
Although we are far from an ideal temporary skin substitute, biological dressings
have shown better functional and aesthetic results6,14. In this
context, Nile tilapia skin is a promising product. Tilapia represents 45.4% of
the total fish production in Brazil, but its skin is a waste byproduct of which
only 1% is used in handicrafts. Tilapia skin must still be subjected to
scientific analyses of its activity in humans. Several studies have compared
human skin with Tilapia skin6,7,14,17-20, and
favorable results were described regarding their histological and histochemical
aspects and tensiometric properties18,20.
In this study, Nile tilapia skin was used in the treatment of 15 patients, 53.3%
affected by second-degree superficial burns and 46.7% by second-degree deep
burns.
To use animal skin as an occlusive dressing, a rigorous disinfection and
sterilization protocol must be followed. Recent research indicates that chemical
sterilization and radiosterilization are effective for the preparation of Nile
tilapia skin18. The skins were provided
by the Center for Research and Development of Medicines of the Federal
University of Ceará, which is responsible for the sterilization processing.
Tilapia skin molds and adheres to the wound, creating a kind of tampon that
prevents contamination and fluid loss.
The results of this study showed that the mean treatment time with Nile tilapia
skin (9.6 ± 2.3 days) was similar to that with Aquacel AG®
(10.7 ± 4.5 days).
Pain during and after the dressing change was assessed using a VAS. Patients in
both groups reported a VAS score > 5 at the time of the initial cleaning and
dressing application process. At the end of the dressing application, 86.7% of
the patients in the Nile tilapia skin group reported feeling less pain, proven
by VAS scores ≤ 5, compared to 46.7% of patients in the Aquacel
AG® group (p = 0.05).
Skins and dressings are changed according to the amount of exudate. However, the
higher the number of exchanges, the higher the risk of infection, the higher the
cost of treatment, and the greater the possibility that the patient will feel
pain. Given these aspects, it should be emphasized that fewer patients treated
with Nile tilapia skin required dressing exchanges. In nine patients (60%)
treated with Nile tilapia skin, there was no need for replacement of any
dressing, while 53.3% of patients treated with Aquacel AG®
required at least one exchange. Thus, considering the p value =
0.71 (p ≥ 0.05), skin healing with Nile tilapia skin was
not inferior to that with Aquacel AG®.
The findings of this study suggest that Nile tilapia skin is as effective as
Aquacel AG® in the management and treatment of second-degree
burns in adults.
CONCLUSIONS
Based on the results of this study, Nile tilapia skin is an effective occlusive
biological dressing in the management and treatment of second-degree burns in
adults. The average treatment time of the patients treated with Nile tilapia
skin (9.6 ± 2.4 days) was similar to that of patients treated with Aquacel
AG® (10.7 ± 4.5 days). Furthermore, no significant
intergroup difference was noted in pain level after dressing or the need for
replacement during treatment.
COLLABORATIONS
|
MJBM
|
Analysis and/or data interpretation, conceptualization, data
curation, funding acquisition, investigation, methodology,
realization of operations and/or trials, writing - original draft
preparation, writing - review & editing.
|
|
CTB
|
Conceptualization, final manuscript approval, formal analysis,
supervision, writing - review & editing.
|
REFERENCES
1. Cruz BF, Cordovil PBL, Batista KNM. Perfil epidemiológico de
pacientes que sofreram queimaduras no Brasil: revisão da literatura. Rev Bras
Queimaduras. 2012;11(4):246-50.
2. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de
Atenção Especializada. Cartilha para Tratamento de Emergência das Queimaduras.
Brasília: Ministério da Saúde; 2012. [acesso 2019 Jan 30]. [Disponível em:
http://bvsms.saude.gov.br/bvs/publicacoes/cartilha_tratamento_emergencia_queimaduras.pdf
3. Ferreira E, Lucas R, Rossi L, Andrade D. Curativo do paciente
queimado: uma revisão de literatura. Rev Esc Enferm USP. 2003;37(1):44-51. DOI:
http://dx.doi.org/10.1590/S0080-62342003000100006
4. Hansbrough JF, Zapata-Sirvent RL, Cooper ML. Effects of topical
antimicrobial agents on the human neutrophil respiratory burst. Arch Surg.
1991;126(5):603-8. PMID: 1850590 DOI: http://dx.doi.org/10.1001/archsurg.1991.01410290079016
5. Chanda J, Rao SB, Mohanty M, Muraleedharan CV, Arthur VL,
Bhuvaneshwar GS, et al. Use of glutaraldehyde-gentamicin-treated bovine
pericardium as a wound dressing. Biomaterials. 1994;15(1):68-70. DOI: http://dx.doi.org/10.1016/0142-9612(94)90200-3
6. Alves APNN, Lima Verde MEQ, Ferreira Júnior AE, Silva PGB, Feitosa
VP, Lima Júnior EM, et al. Avaliação microscópica, estudo histoquímico e análise
de propriedades tensiométricas da pele de tilápia do Nilo. Rev Bras Queimaduras.
2015;14(3):203-10.
7. Franco MLRS, Franco NP, Gasparino E, Dorado DM, Prado ME, Vesco APD.
Comparação das peles de tilápia do nilo, pacu e tambaqui: Histologia, composição
e resistência. Arch Zootec. 2013;62(237):21-32. DOI: http://dx.doi.org/10.4321/S0004-05922013000100003
8. Chem WY, Rogers AA, Lydon MJ. Characterization of biologic
properties of wound fluid collected during early stages of wound healing. J
Invest Dermatol. 1992;99(5):559-64. DOI: http://dx.doi.org/10.1111/1523-1747.ep12667378
9. Rajanbabu V, Chen JY. Applications of antimicrobial peptides from
fish and perspectives for the future. Peptides. 2011;32(2):415-20. DOI:
http://dx.doi.org/10.1016/j.peptides.2010.11.005
10. Hunag PH, Chen JY, Kuo CM. Three different hepcidins from tilapia,
Oreochromis mossambicus: analysis of their expressions and biological functions.
Mol Immunol. 2007;44(8):1922-34. DOI: http://dx.doi.org/10.1016/j.molimm.2006.09.031
11. Costa GOP, Silva JA, Santos AG. Perfil clínico e epidemiológico das
queimaduras: evidências para o cuidado de enfermagem. Ciênc Saúde.
2015;8(3):146-55.
12. Farina JR JA. Novas tecnologias no tratamento de queimaduras. Rev
Plastiko's. 2018:47-9.
13. Moser H, Pereima RR, Pereima MJL. Evolução dos curativos de prata no
tratamento de queimaduras de espessura parcial. Rev Bras Queimaduras.
2013;12(2):60-7.
14. Miranda MJB. Viabilidade da pele da Tilápia-do-Nilo (Oreochromis
niloticus). An Fac Med Olinda. 2018;1(1):49-52.
15. Hayneman A, Hoeksema H, Vandekerckhove D, Pirayesh A, Monstrey S.
The role of silver sulphadiazine in the conservative treatment of partial
thickness burn wounds: A systematic review. Burns. 2016;42(7):1377-86. DOI:
http://dx.doi.org/10.1016/j.burns.2016.03.029
16. Tavares WS, Silva RS. Curativos utilizados no tratamento de
queimaduras: uma revisão integrativa. Rev Bras Queimaduras.
2015;14(4):300-6.
17. Lima Junior EM, Bandeira TJPG, Miranda MJB, Ferreira GE, Parente EA,
Piccolo NS, et al. Characterization of the microbiota of the skin and oral
cavity of Oreochromis niloticus. J Health Biol Sci.
2016;4(3):193-7.
18. Alves APNN, Lima Júnior EM, Piccolo NS, de Miranda MJB, Lima Verde
MEQ, Ferreira Júnior AEC, et al. Study of tensiometric properties,
microbiological and collagen content in nile tilapia skin submitted to different
sterilization methods. Cell Tissue Bank. 2018;19(3):373-82.
19. Lima Júnior EM. Tecnologias inovadoras: uso da pele da tilápia do
Nilo no tratamento de queimaduras e feridas. Rev Bras Queimaduras.
2017;16(1):1-2.
20. Lima-Júnior EM, Picollo NS, Miranda MJB, Ribeiro WLC, Alves APNN,
Ferreira GE, et al. Uso da pele de tilápia (Oreochromis niloticus), como
curativo biológico oclusivo, no tratamento de queimaduras. Rev Bras Queimaduras.
2017;16(1):10-7.
1. Universidade Federal de Pernambuco, Recife, PE,
Brazil.
Corresponding author: Marcelo José Borges de
Miranda Avenida Boa Viagem, nº 3296/102 - Boa Viagem, Recife, PE,
Brazil Zip Code 51020-001 E-mail:
mborgesmais@hotmail.com
Article received: October 30, 2018.
Article accepted: November 11, 2018.
Conflicts of interest: none.