

Original Article - Year 2019 - Volume 34 -
Carpal tunnel syndrome associated with amyloidosis
Síndrome do túnel do carpo associada à amiloidose
ABSTRACT
Introduction: Amyloidosis features protein deposition in the organs and tissues and has been associated with carpal tunnel syndrome (CTS) when it occurs in the wrist. The objective is to describe a case series of patients undergoing surgery for CTS associated with amyloidosis.
Methods: The study included 12 patients who underwent surgery to treat CTS in whom amyloidosis was proven by biopsy; the follow-up period was 5 years. The patients were evaluated by clinical tests, electroneuromyography, radiological images, and biopsy.
Results: All patients presented with musculoskeletal complaints, severe symptoms of median nerve compression, and changes on neurophysiological tests. Surgery, synovectomy, and biopsy were performed. In the postoperative period, five patients (41%) developed chronic pain and reflex sympathetic dystrophy.
Conclusion: A higher frequency of postoperative pain was observed in the patients, demonstrating the need for caution in the approach and treatment of this association.
Keywords: Amyloidosis; Carpal tunnel syndrome; Reconstructive surgical procedures; Orthopedic procedures; Delivery of health care
RESUMO
Introdução: A amiloidose é caracterizada pela deposição de proteínas nos órgãos e tecidos, e tem sido associada à síndrome do túnel do carpo (STC) quando ocorre no punho. O objetivo é descrever uma série de casos de pacientes submetidos à cirurgia para STC associado à amiloidose.
Métodos: O estudo incluiu 12 pacientes que se submeteram à cirurgia para tratar a STC cuja biópsia identificou amiloidose; o seguimento foi de cinco anos. Os pacientes foram avaliados por testes clínicos, eletroneuromiografia, imagens radiológicas e biópsia.
Resultados: Todos os pacientes apresentaram queixas musculoesqueléticas, sintomas severos de compressão do nervo mediano, alterações nos testes neurofisiológicos. Realizou-se a cirurgia, sinovectomia e biópsia. No pós-operatório, cinco pacientes (41%) desenvolveram dor crônica e distrofia simpático-reflexa.
Conclusão: Observou-se maior frequência de dor pós-operatória na amostra, o que revela a necessidade de atenção na abordagem e tratamento dessa associação.
Palavras-chave: Amiloidose; Síndrome do túnel carpal; Procedimentos cirúrgicos reconstrutivos; Procedimentos ortopédicos; Assistência à saúde
INTRODUCTION
Amyloidosis is the deposition of abnormal proteins and accumulation of insoluble amyloid fibrils in the tissues and organs1-3. This process occurs through the aggregation of proteins, production of ordered polymers, and formation of protofilaments and fibrils. Recent theories have suggested that the lesions result from multiple mechanisms, not only fibrin deposition but also the precursor structures (transthyretin, apolipoproteins, insulin, prion proteins, lysozyme, cystatin C) that interact with the affected cells2,3.
A disease-related mutation in the TTR gene has been described. Associations with pathological inflammatory states and neoplastic and hereditary factors have been identified. Some of these associations do not seem to have clinical impacts. However, the criteria for amyloidosis include age > 50 years, chronic infection or inflammatory disease, family history of amyloidosis, multiple myeloma, renal disease, and dialysis.
Nevertheless, depending on fibril deposition amount and location, the disease may be more aggressive, affecting any organ, including the kidneys, heart, and nerves1-3. Different types of amyloidosis have been classified and described, including primary, secondary, familial, microglobulin beta 2, and localized.
The association between amyloidosis and carpal tunnel syndrome (CTS) has a prevalence of 2–8%4-6. This association is usually followed by hemodialysis or familial amyloidosis. The clinical diagnosis of CTS includes paresthesia, pain, weakness in the hands, and thenar atrophy. Nerve conduction studies by electroneuromyography are useful for detecting the peripheral nerve involvement present in cases of neuropathic amyloidosis7-9.
The gold standard diagnostic method for amyloidosis is a biopsy of the affected tissue after staining with hematoxylin-eosin and Congo red. Although median nerve compression can be caused by extrinsic and intrinsic factors, amyloid deposition alone can cause nerve compression, being an already established association; however, the clinical evolution of the operated cases merits study4,6.
METHODS
A total of 807 patients underwent CTS surgery at our institution over a 5-year interval; of them, 436 underwent biopsy and synovectomy. This procedure was performed in accordance with the transoperative findings of synovial thickening. The removed synovial tissue was sent for histopathological study, which revealed that 12 (3%) patients tested positive for amyloidosis.
This was a retrospective study that included analysis of these patients’ medical records with respect to their clinical findings, history, comorbidities, diagnostic tests performed, and postoperative follow-up. The study was submitted and approved by the CEP/APS Ethics Committee (CAEE no. 53326916.2.0000.0022).
Pre-operative evaluation
The patients were clinically evaluated for symptoms of paresthesia, numbness, tingling, and pain. The physical assessment included Tinel’s test, Phalen’s test, the Semmes–Weinstein monofilament test, the short thumb abductor and opponens pollicis rating scale (0–5, such as the British Medical Research Council Scale), and dynamometer force measurements.
Complementary examinations, such as electroneuromyography of the limbs, radiography of the wrist, and magnetic resonance imaging, were also performed. The severity criteria for CTS were classified according to the neurophysiological rating scale described by Bland (Chart 1)10. The diagnosis of reflex sympathetic dystrophy was made in accordance with International Association for the Study of Pain guidelines8. The surgeries were performed in the Departments of Plastic Surgery and Orthopedics of our institution, and the surgeons performed the pre- and post-operative ratings.
| Degree | |
|---|---|
| 0 | Normal |
| I | Very mild; demonstrable only with more sensitive tests |
| II | Mild; sensory nerve conduction slow velocity, normal motor latency |
| III | Moderate; sensory potential preserved with motor slowing, motor latency of the abductor pollicis brevis (APB) < 6.5 ms |
| IV | Severe; sensory potential absent, but motor response preserved, distal motor latency to APB < 6.5 ms |
| V | Very severe; terminal latency to APB > 6.5 ms |
| V | Extremely severe; sensory and motor potentials unrecordable (surface motor potential from APB < 0.2 mV amplitude) |
Surgical procedure
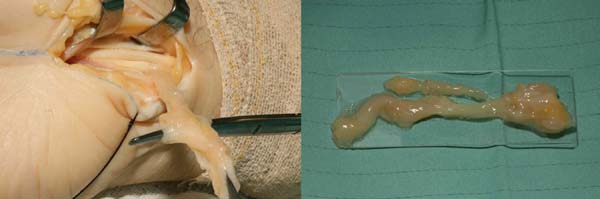
All procedures were performed under general anesthesia or a regional brachial plexus block. Tourniquets were used in the arm after exsanguination of the limb with a systolic pressure of 100–120. The classic open volar incision was used to release the carpal tunnel and the fascia of the forearm. The median nerve and flexor tendon sheath were inspected (Figure 1). During surgery, the flexor tendon sheath and transverse carpal ligament were biopsied when thickening and macroscopic changes were evident (Figure 1).
The histopathological studies included staining with hematoxylin-eosin and Congo red, and the assessment of amyloid deposits was performed using polarized light (Figure 2). Chart 1 summarizes the disease severity.
RESULTS
Charts 2 and 3 describe the clinical and demographic distribution of the 12 patients (nine females, three males). Eleven patients were >50 years of age. The time between the onset of the initial symptoms and surgery ranged from 6 months to 17 years; 11 patients had the disease for more than 1 year; and the follow-up period was 1–9 years.
| Grading of symptoms | |
| Grade I | Intermittent symptoms of pain and paresthesia, nocturnal paresthesia in the distribution of the median nerve, motor and sensory exam normal. |
| Grade II | Constant symptoms with a reduction of the fine pincer grasp and sensory alterations in the Semmes-Weinstein Monofilament test with weakness of the thenar muscles. |
| Grade III | Extensive sensory loss and atrophy of the thenar muscles. |
| Perioperative findings | |
| I - Normal | Thickening and flattening of the nerve with normal vascularization and structures of the epineurium, without fibrosis. |
| II - Moderate | Moderate decrease in vascularization, mild to moderate fibrosis in some part of the nerve, mild to moderate synovial thickening. |
| III - Severe | Loss of vascularization, diffuse fibrosis around the nerve, large synovial thickening, and appearance of pseudoneuroma. |
| Histopathological findings | Fibrosis, thickening of the collagen bundles, hyaline degeneration, proliferation of the synovia, edema and vascular lesions, amyloid deposits. |
| Age | Sex | Musculoskeletal change | Clinical score | EMG score | Surgical score | Time between symptom onset and treatment | Follow-up | Postoperative evolution |
|---|---|---|---|---|---|---|---|---|
| 38 | F | Wrist, elbow, and shoulder | III | 6 | III | 17 years | 9 years | Dystrophy |
| 74 | F | Elbow and shoulder | II | 6 | II | 2 years | 3 years | Dystrophy |
| 72 | F | Knee | II | 5 | III | 2 years | 6 years | Normal |
| 62 | F | Cervical spine | III | 5 | III | 1 year | 3 years | Improvement |
| 71 | M | Shoulder | III | 5 | II | 6 months | 4 years | Improvement |
| 56 | F | Tenosynovitis | III | 5 | III | 2 years | 1 year | Dystrophy |
| 48 | M | Tenosynovitis | III | 5 | I | 1 year | 3 years | Chronic pain and atrophy |
| 50 | F | Cervical spine | I | 5 | II | 2 years | 5 years | Normal |
| 64 | M | Column and polyarthralgia | II | 5 | III | 1 year | 1 year | Normal |
| 53 | F | Cervical and dorsal | III | 5 | III | 3 years | 6 years | Improvement |
| 73 | F | Tenosynovitis | III | 5 | III | 5 years | 1 year | Improvement |
| 72 | F | Shoulder, knee, and cervical | III | 5 | III | 5 years | 5 years | Neuropathic pain, polyneuropathy |
All patients had associated musculoskeletal complaints, such as polyarticular pain, stenosing tenosynovitis, and shoulder bursitis. One case of associated polyneuropathy with no family history was found. Some erythrocyte sedimentation rates were slightly elevated (26–27 mm in the first hour). Only one patient presented radiographic changes in the wrist that were associated with a history of trauma.
Six patients had degenerative changes in the joints (cervical, shoulder, or knee). No patient had a history of cancer or hemodialysis. However, two patients had a family history of intestinal and brain cancer. One man reported acute myocardial infarction. One woman presented with CTS and the deposition of adipose tissue and previous trauma of the wrist associated with radiocarpal joint degeneration.
In this series of 12 patients, five (41.6%) had complex regional pain and symptom recurrence after the carpal tunnel release. Three patients had difficulty controlling reflex sympathetic dystrophy with the recommended treatment. All cases presented CTS associated with flexor tenosynovitis and a localized form of amyloidosis.
The prevalence of chronic pain as a complication after the surgical procedure was 45.5%; consequently, analgesics including paracetamol, gabapentin, amitriptyline, codeine, and corticosteroids were administered. Among the five patients who presented with this undesirable surgical complication, three were diagnosed with reflex sympathetic dystrophy, one with neuropathic pain, and one with effort-related pain.
Synovectomy was performed in all cases of this series, although epineurolysis was not necessary and not performed in any case because no neural invasion by the amyloidotic tissues was observed. Instead, the flexor sheath was invaded by these tissues. As there was no cleavage plan to remove all tissues, a partial synovectomy was used to reduce local volume and enable the histopathological examination.
DISCUSSION
The association between amyloidosis and peripheral neuropathy was reported for the first time by De Navasquez in 1938 andKyle et al.6. Several proteins and its precursors have been implicated in amyloidosis (transthyretin, apolipoprotein AI, apolipoprotein AII, cystatin C, gelsolin, fibrinogen alpha chain, lysozyme, and β2 microglobulin (Aβ2-M)2,11-16. In addition, more than 80 transthyretin mutations have been described, and evidence indicates that the fibrinogen and other protein aggregations perform an important role in the pathogenesis of CTS17.
The main clinical manifestations and syndromes associated with neuropathy are related to light-chain or primary amyloidosis and familial forms of the autosomal dominant disease2,13. The diagnosis of amyloidosis is usually made at approximately 60 years of age with a male predominance of 2:12,4,5. The most common presentation is peripheral neuropathy, although CTS can occur without alterations in the skin, proteinuria, or skeletal manifestations (degenerative arthropathy, chronic arthralgia, or arthritis)16-20.
CTS may be associated with different types of amyloidosis, especially their primary forms, in patients on chronic hemodialysis14,18,21. In this disease, the first stages of nerve compression involve progressive ischemia and a local inflammatory process. During the gradual compressive process, demyelination occurs and nerve conduction velocity decreases in the area of compression.
Our study found that all patients had axon loss, demyelination, low focal conduction, and signs of denervation, which are classified as grade V electroneuromyography findings. For these patients, a differential diagnosis, including radiculopathy, motor neuron disease, polyneuropathy, ulnar neuropathy, and other deposition diseases such as gout and rheumatoid arthritis, is important.
Virchow introduced the first histochemical diagnostic test for amyloidosis, later perfected by Benhold (1922) with Congo red staining that emits a light apple green fluorescence under polarized light4,5. This test is considered the gold standard for the diagnosis of amyloidosis6. Based on the literature, this test was used to confirm the disease in our series of cases, and an additional electronic microscopy assessment was performed.
On electronic microscopy, major and minor fibrillar components were identified that presented a pentamer aspect, known as P component8. We believe that complementary clinical and electrophysiological tests are important for the early diagnosis of CTS, the identification of amyloidosis, and an early therapeutic plan. We recognize the challenges of grading the symptoms, signs, and surgical findings since their accuracy depends on clinician experience and knowledge of the natural characteristics of the disease.
During the clinical follow-up, the cohort showed a high prevalence of chronic pain compared with patients with idiopathic CTS9. Based on the experience of the authors, chronic pain was controlled more effectively by combining analgesics and non-steroidal and steroidal anti-inflammatory agents. Some surgeons have postulated that chronic postoperative pain can be related with the anesthetic technique or tourniquet used.
Nevertheless, another study performed at our institution did not demonstrate this association22. We believe that the systemic disease (amyloidosis) can influence peripheral nerve recovery after surgical trauma and may even increase the cases of chronic pain. The risk factors for the association between amyloidosis and CTS may not have been well evaluated in this study due to the small sample size and the limitations of retrospective cases series.
Patients with a long history of compressive symptoms of the median nerve, associated with severe changes on an EMG examination, complaints, musculoskeletal disorders, and a history of myocardial infarction, hemodialysis, and family history of neuropathy, are indications for intraoperative biopsy during the surgical nerve decompression and amyloidosis assessment.
The authors believe that the poor result may have occurred for various reasons such as the fact that the patients had a severe form of CTS before surgery, as indicated by clinical scores, EMG, and surgery; and associated articular diseases. Although one of the limitations of the present study our inability to assess risk factors between amyloidosis and CTS since the 12 evaluated patients had equally poor postoperative courses, it could also have been seen for a variety of other reasons, or simply been a group of most severely affected patients regardless of whether they had amyloid deposits.
However, our findings call attention to this group of patients with poor preoperative presentation possibly associated with amyloidosis and worse postoperative prognosis. This study’s findings emphasize the importance of this association and the need for more research to increase our understanding of the risk factors and consequences of the association between peripheral nerve disease and amyloidosis.
CONCLUSION
This infrequent association affected the results of this case study, with a higher prevalence of postoperative pain. The strategies adopted for treating patients with CTS associated with amyloidosis in our institution include the assessment of amyloidosis when treating patients with other musculoskeletal complaints, especially those older than 60 years of age who present with symptoms of CTS, thenar atrophy, and electroneuromyography grade V findings. We suggest treating these patients with early nerve decompression, synovectomy, assessing amyloid deposits, and effectively controlling postoperative pain.
COLLABORATIONS
|
KTB |
Conception and design study, data curation, project administration. |
|
GCSA |
Analysis and/or data interpretation, final manuscript approval, writing - original draft preparation. |
|
UPS |
Investigation, methodology, supervision. |
|
GBM |
Methodology, project administration, writing - review & editing. |
REFERENCES
1. Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1(6):1331-41. DOI: https://doi.org/10.2215/CJN.02740806
2. Seldin DC, Skinner M. Amyloidosis. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. New York: McGraw Hill Education; 2015. p. 945-50.
3. Raivio VE, Jonasson J, Myllykangas L, Ala-Mello S, Kankuri-Tammilehto M, Kiuru-Enari S, et al. A novel transthyretin Lys70Glu (p.Lys90Glu) mutation presenting with vitreous amyloidosis and carpal tunnel syndrome. Amyloid. 2016;23(1):46-50. DOI: https://doi.org/10.3109/13506129.2015.1126574
4. Kyle RA, Greipp PR. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983;58(10):665-83. PMID: 6353084
5. Benson MD. The hereditary amyloidoses. Best Pract Res Clin Rheumatol. 2003;17(6):909-27. DOI: https://doi.org/10.1016/j.berh.2003.09.001
6. Kyle RA, Eilers SG, Linscheid RL, Gaffey TA. Amyloid localized to tenosynovium at carpal tunnel release. Natural history of 124 cases. Am J Clin Pathol. 1989;91(4):393-7. DOI: https://doi.org/10.1093/ajcp/91.4.393
7. Niemczyk S, Was M, Gomólka M. Carpal tunnel syndrome in dialysed patients - interdisciplinary experiences. Ortop Traumatol Rehabil. 2004;6(3):367-72.
8. Tuncali D, Barutcu AY, Terzioglu A, Aslan G. Carpal tunnel syndrome: comparison of intraoperative structural changes with clinical and electrodiagnostic severity. Br J Plast Surg. 2005;58(8):1136-42. DOI: https://doi.org/10.1016/j.bjps.2005.05.010
9. Afshar A, Yekta Z, Mirzatoluei F. Clinical course of the non-operated hand in patients with bilateral idiopathic carpal tunnel syndrome. J Hand Surg Am. 2007;32(8):1166-70. DOI: https://doi.org/10.1016/j.jhsa.2007.06.003
10. Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23(8):1280-3. DOI: https://doi.org/10.1002/1097-4598(200008)23:8<1280::AID-MUS20>3.0.CO;2-Y
11. Borman H, Akinbingol G, Maral T, Sozay S. Entrapment neuropathy of the upper extremity in hemodialysis patients. Plast Reconstr Surg. 2002;109(7):2598-9. DOI: https://doi.org/10.1097/00006534-200206000-00078
12. Assmus H, Staub F. Recurrences of carpal tunnel syndrome in long-term haemodialysis patients. Handchir Mikrochir Plast Chir. 2005;37(3):158-66. PMID: 15997426 DOI: https://doi.org/10.1055/s-2005-837699
13. Liu YT, Lee YC, Yang CC, Chen ML, Lin KP. Transthyretin Ala97Ser in Chinese-Taiwanese patients with familial amyloid polyneuropathy: genetic studies and phenotype expression. J Neurol Sci. 2008;267(1-2):91-9. PMID: 18022643
14. Yamamoto S, Gejyo F. Historical background and clinical treatment of dialysis-related amyloidosis. Biochim Biophys Acta. 2005;1753(1):4-10. PMID: 16226497 DOI: https://doi.org/10.1016/j.bbapap.2005.09.006
15. Breda S, Richter HP, Schachenmayr W. Incidence of biopsy-detectable amyloid deposits in the retinaculum flexorum and in the tenosynovial tissue in carpal tunnel syndrome. Zentralbl Neurochir. 1993;54(2):72-6. PMID: 8368037
16. Shin SC, Robinson-Papp J. Amyloid neuropathies. Mt Sinai J Med. 2012;79(6):733-48. PMID: 23239211 DOI: https://doi.org/10.1002/msj.21352
17. Utrobičić I, Novak I, Marinović-Terzić I, Matić K, Lessel D, Salamunić I, et al. Carpal tunnel syndrome is associated with high fibrinogen and fibrinogen deposits. Neurosurgery. 2014;75(3):276-85. DOI: https://doi.org/10.1227/NEU.0000000000000422
18. Drüeke TB. Extraskeletal problems and amyloid. Kidney Int Suppl. 1999;73:S89-93.
19. Janunger T, Anan I, Holmgren G, Lövheim O, Ohlsson PI, Suhr OB, et al. Heart failure caused by a novel amyloidogenic mutation of the transthyretin gene: ATTR Ala45Ser. Amyloid. 2000;7(2):137-40. DOI: https://doi.org/10.3109/13506120009146252
20. Wilbourn AJ. The electrodiagnostic examination with peripheral nerve injuries. Clin Plast Surg. 2003;30(2):139-54. DOI: https://doi.org/10.1016/S0094-1298(02)00099-8
21. Kurer MH, Baillod RA, Madgwick JC. Musculoskeletal manifestations of amyloidosis. A review of 83 patients on haemodialysis for at least 10 years. J Bone Joint Surg Br. 1991;73(2):271-6. PMID: 2005153 DOI: https://doi.org/10.1302/0301-620X.73B2.2005153
22. da Costa VV, de Oliveira SB, Fernandes Mdo C, Saraiva RA. Incidence of regional pain syndrome after carpal tunnel release. Is there a correlation with the anesthetic technique? Rev Bras Anestesiol. 2011;61(4):425-33.
1. Rede Sarah de Hospitais de Reabilitação,
Brasília, DF, Brazil.
Corresponding author: Katia Torres Batista SMHS 501 bloco A , Brasília, DF, Brazil Zip Code 70335-901 E-mail: katiatb@terra.com.br
Article received: October 23, 2018.
Article accepted: February 10, 2019.
Conflicts of interest: none.







 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter