INTRODUCTION
The use of cutaneous, fasciocutaneous, muscle, free tissue transfer, and various
other flaps have been described to repair the loss of substance in the medium
and distal thirds of the leg; several authors consider that the treatment of
these defects as a unique challenge1-5. Exposure of
the bones, tendons, and osteosynthesis materials associated with the lack of
availability of local soft tissue in this area of the body are contributing
factors that hinder planning and therapy in such cases2.
Free microsurgical transfer flaps are often the first therapeutic option to
promote adequate coverage; their use requires a specific professional
multidisciplinary team, specialized infrastructure, and higher cost and must
be
reserved for traumas that compromise the posterior muscle compartment and
vascularization of the leg1-6. However,
cutaneous, fasciocutaneous, and even fasciosubcutaneous flaps are technically
more accessible, have higher rotational arcs, lower donor-site morbidity rates,
and simpler postoperative courses,3,5,7-9 but they must
be avoided in situations that require the provision of an increased local blood
supply (e.g., fractures with exposed bone)1.
When local muscle flaps are carefully indicated and implemented, they can provide
rich vasculature coverage and intermediate thickness6. The main muscles used for medial- and distal-third
reconstruction include the flexor digitorum longus, tibialis posterior, flexor
hallucis longus, peroneus brevis, peroneus longus, extensor digitorum longus,
extensor hallucis longus, reverse tibialis anterior, and soleus4,10,11. Limitations
and probable contraindications include patients with severe muscle and
vascular-associated traumas and the presence of peripheral vascular disease1,6.
Soleus flaps were first described by Magee et al. in 198012. In 1985, Tobin13
systematized and correlated the morphology of the soleus muscle with the
vascular pattern of its pedicles and intramuscular and extramuscular branches,
allowing the creation of a large pediculated flap made with the longitudinal
half of the hemisoleus flap. This flap can be constructed using the medial or
lateral belly of the soleus muscle using direct or reverse blood flow.
Therefore, four flaps are possible: medial hemisoleus pediculated proximally,
medial hemisoleus pediculated distally (or reverse), lateral hemisoleus
pediculated proximally, and lateral hemisoleus pediculated distally (or
reverse)13. The main advantage of
these flaps is the preservation of the innervation of the half of the soleus
muscle that remains in the donor site, which maintains the plantar flexion
force. In addition, the hemisoleus flap has a larger rotational arc than the
conventional soleus flap13-15.
In 2005 and 2008, Pu described surgical refinement techniques establishing
parameters and increasing reliability for medial hemisoleus flaps6,16. More recently, the association of the technique with angiosome
principles (three-dimensional tissue blocks whose irrigation is provided by a
branch of a specific deep stem artery)17
described by Taylor & Palmer (1987)17
the design of the flap and the need for careful dissection with the benefit of
obtaining a thinner flap with lower donor-site morbidity rates, especially for
reverse flow flaps1,9,18.
The medial hemisoleus flap is the most frequently used because of its proximity
to the defects that occur more frequently in the three-quarter proximal tibia
and because it presents a greater rotation arc than the lateral hemisoleus
flap1,6,13.
OBJECTIVE
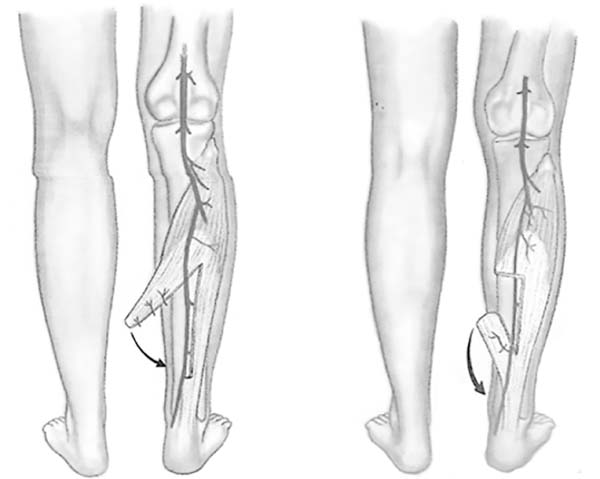
This study aimed to report the use of the medial hemisoleus flap proximal (direct
flow) or distal (reverse flow) (Figure 1),
emphasize its advantages, confirm its indications, and analyze the complications
of this procedure as an alternative in the surgical arsenal to repair substance
losses of the medial and distal thirds of the leg.
Figure 1 - Possible muscle flaps: direct or reverse flow.
Figure 1 - Illustration: Arantes HL, Freitas AG, Figueiredo JCA. Muscle and
musculocutaneous patchwork. In Mélega JM. Plastic Surgery -
fundamentals and art - general principles. Rio de Janeiro: MEDSI;
2002. p.136.
Figure 1 - Possible muscle flaps: direct or reverse flow.
Figure 1 - Illustration: Arantes HL, Freitas AG, Figueiredo JCA. Muscle and
musculocutaneous patchwork. In Mélega JM. Plastic Surgery -
fundamentals and art - general principles. Rio de Janeiro: MEDSI;
2002. p.136.
METHODS
Over a 10-year period, a total of nine medial hemisoleus flaps were created in
eight patients (six men, two woman) to repair substance losses of traumatic
etiology with tibial exposure. The surgeries were performed at the Ibiapaba
Cebams Hospital of Barbacena - MG. The Medical Ethics Committee of Ibiapaba
Cebams Hospital analyzed and approved this study (protocol number 001/2018).
The inclusion criteria were as follows:
Presence of a wound with exposed bone or osteosynthesis material in
the medial or distal third of the leg comprising an area < 50
cm².
Stabilization of the fracture when present.
Lack of peripheral vascular disease or other conditions that could
compromise the vascular pattern of the soleus muscle, such as
diabetes, smoking, paraplegia, or previous history of vascular
obstruction of the lower limb.
Lack of trauma evidence of compromise of the medial muscle belly of
the soleus.
Treatment of established infectious condition with debridement,
dressings, and systemic antibiotic therapy before the procedure.
The soleus muscle has a characteristic bipeniform morphological pattern (98.8% of
cases) in which the medial and lateral belly present independent neurovascular
supplies and are longitudinally separated at the medial line. The medial belly
originates in the proximal tibia and inserts into the dorsomedial aspect of the
calcaneal tendon, while the lateral belly originates in the proximal fibula and
inserts into the dorsolateral side of the calcaneal tendon. In the distal half,
the medial and lateral bellies of the muscle are separated longitudinally by
an
intramuscular septum; in the proximal half, these same bellies are fused13.
The vascularization is type II Mathes & Nahai19. There are two proximal dominant pedicles: the tibialis posterior
branch that nourishes the medial belly through segmental branches and the
fibular branch that nourishes the lateral belly through proximally segmental
branches and distally axial segmental branches1,6. A distal
perforating branch of the tibialis posterior artery near the medial malleolus
also nourishes the distal portion of the medial belly and forms the base of the
medial reverse hemisoleus flap6.
The medial and lateral bellies of the soleus muscle are independently
innervated.
The medial branch of the medial (superficial) popliteal nerve and the medial
branch of the tibialis posterior nerve (motor) innervate the medial belly of
the
soleus, whereas the lateral belly of the soleus is innervated by the lateral
branches of the medial popliteal and posterior tibial nerves13.
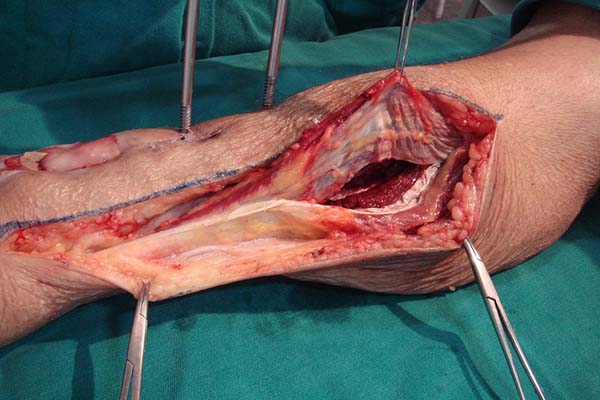
Surgical technique
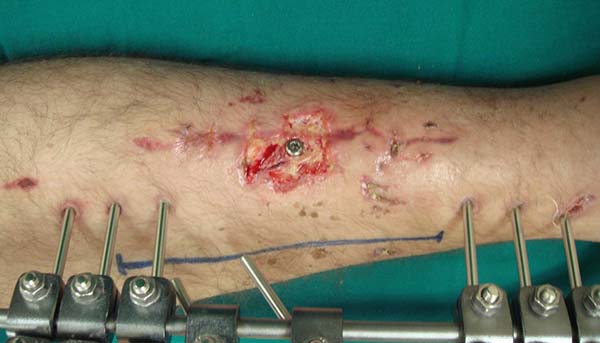
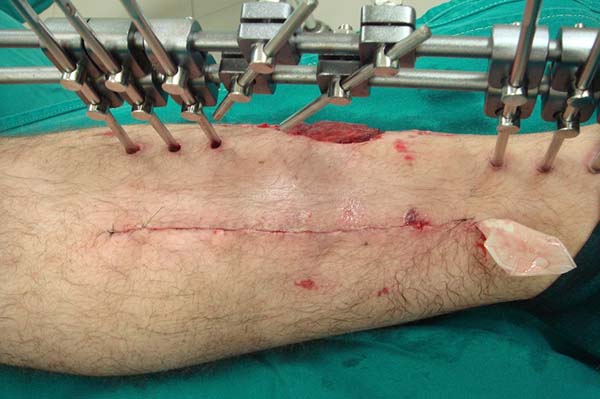
A longitudinal cutaneous incision is made on the medial side of the leg
parallel to the medial border of the tibia. The existence of external
orthopedic fixators should be considered an inherent part of this surgical
procedure, since they are present in most cases (Figure 2). The skin is carefully retracted and allows
opening and exposure of the subcutaneous and fascial planes. Whenever
possible, superficial nerves (sural, saphenous) and vessels (saphenous vein)
should be preserved1. The
gastrocnemius muscle is separated from the medial portion of the soleus
muscle by blunt dissection20, the
deep fascia is opened, and the soleus border is detached from the tibia with
the scalpel. The major and secondary vascular pedicles are identified.
Careful dissection of the pedicles enables a larger arc of rotation and,
consequently, greater flap reach.
Figure 2 - Marking for the programmed longitudinal incision on the
medial side of the lower leg.
Figure 2 - Marking for the programmed longitudinal incision on the
medial side of the lower leg.
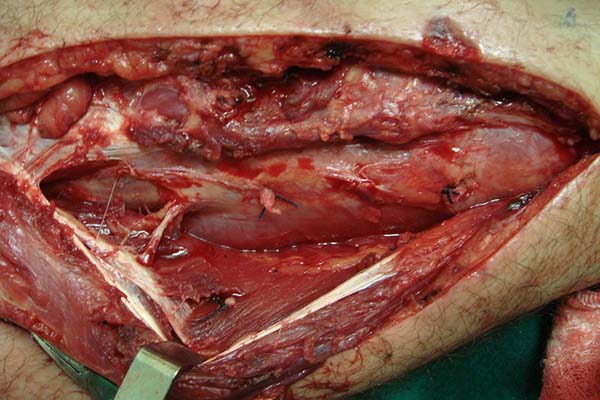
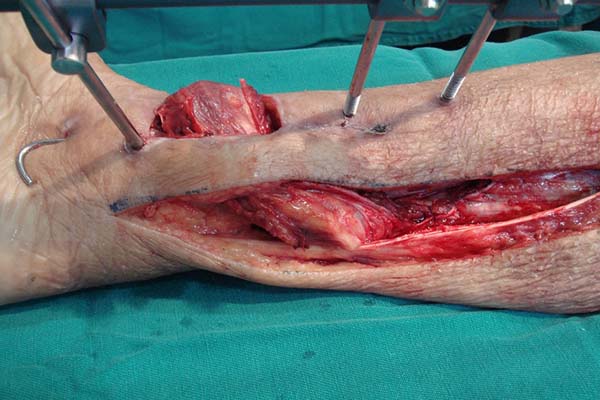
In the preparation of the proximal pedicle medial hemisoleus flap, the main
pedicle (proximal) is maintained and the secondary pedicles are sectioned
(Figure 3). The medial hemisoleus
is then disinserted from the calcaneal tendon using a scalpel or other acute
cutting instrument featuring lower trauma such as those described by Pu6, and the separation of the medial and
lateral bellies of the muscles is guided by the intramuscular septum (Figure 4).
Figure 3 - Sectioning of the distal pedicles and preservation of the
main proximal pedicle.
Figure 3 - Sectioning of the distal pedicles and preservation of the
main proximal pedicle.
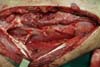
Figure 4 - Muscle disinserted from the calcaneal tendon and separated
from the lateral belly.
Figure 4 - Muscle disinserted from the calcaneal tendon and separated
from the lateral belly.
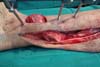
In the preparation of the reverse medial hemisoleus flap, the main pedicle is
maintained (distal perforating branch of the posterior tibial artery) and
the proximal main pedicle and other secondary pedicles are sectioned.
According to the angiosome principle1,8, the flap
will be sectioned 2–3 cm proximal to the connected secondary pedicle since
the next pedicle must be the distal perforating branch of the posterior
distal artery. Separation of the medial and lateral muscle bellies is also
performed under intramuscular septum guidance (Figure 5).
Figure 5 - Separation of the medial and lateral bellies in the creation
of a reverse flap.
Figure 5 - Separation of the medial and lateral bellies in the creation
of a reverse flap.
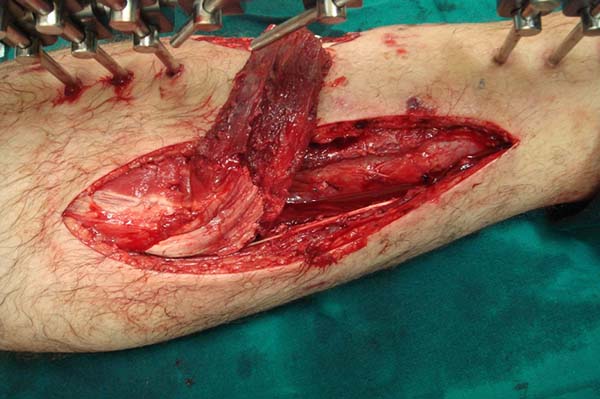
The flap is then rotated under a previously made fasciocutaneous tunnel
(Figures 6 and 7) and the area of the defect is fixed
with a 4.0 absorbable suture. The fasciocutaneous tunnel cannot overcompress
the flap. In this case, a transverse fasciotomy can be performed in the
tunnel and even the communication of the access area to the flap with the
surgical wound.
Figure 6 - Flap rotated under a fasciosubcutaneous tunnel to cover the
wound.
Figure 6 - Flap rotated under a fasciosubcutaneous tunnel to cover the
wound.
Figure 7 - Reverse flap rotated under a fasciocutaneous tunnel.
Figure 7 - Reverse flap rotated under a fasciocutaneous tunnel.
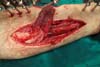
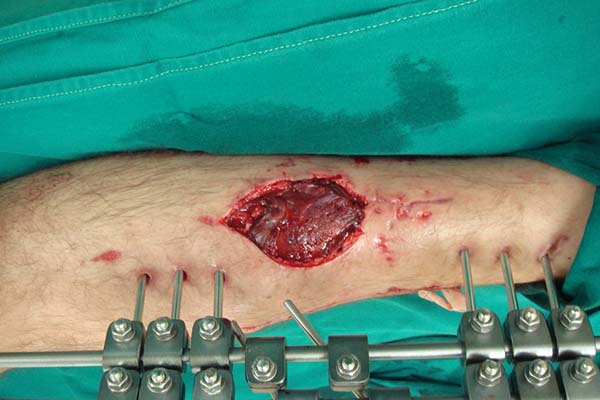
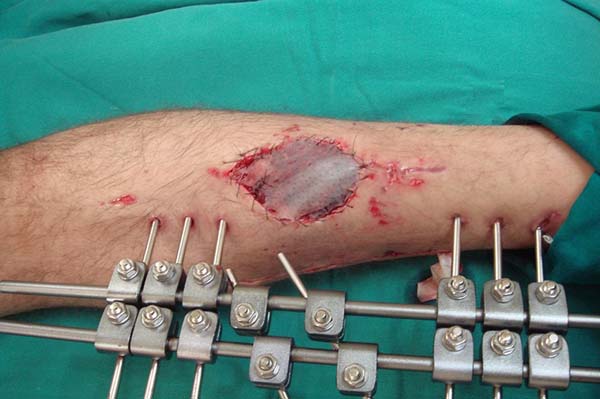
Once fixed in place, the flap is covered with a thin partial-thickness skin
graft (Figure 8). The donor area of
the flap does not require a cutaneous graft and receives a primary suture of
the deep fascia and the subcutaneous tissue with polyglecaprone 4.0 strands.
The skin suture is made using 4.0 nylon thread (Figure 9). Suction drains are used and the
non-compressive dressing generally dispenses with immobilization by a gypsum
chute due to the presence of the external fasteners.
Figure 8 - Partial skin graft over the muscular flap.
Figure 8 - Partial skin graft over the muscular flap.
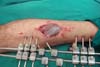
Figure 9 - Aspect of the donor area after suturing.
Figure 9 - Aspect of the donor area after suturing.
In the postoperative period, it is important that the patient remains in bed
with the operated limb elevated for 4–5 days to reduce edema and venous
congestion.
RESULTS
In this series of eight patients (six men [75%] and two women [25%]), nine
hemisoleus flaps were made (Table 1). One
of the patients (Table 1: APO) had
exposed fractures of the bilateral tibiae and underwent bilateral repair with
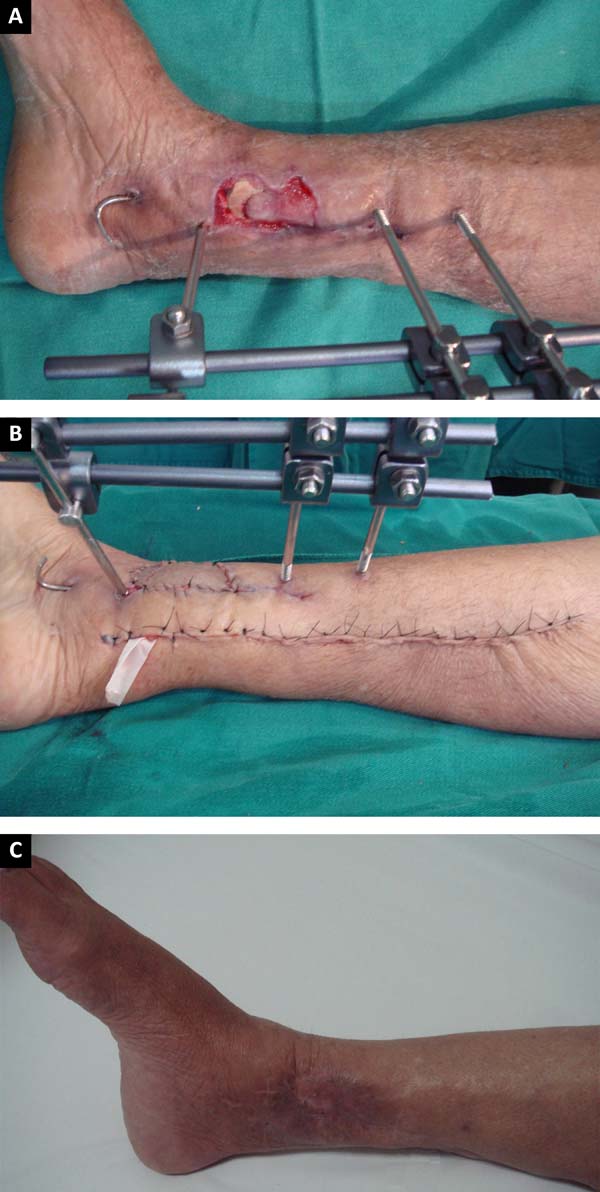
medial hemisoleus flaps at different times. In three patients, a medial reverse
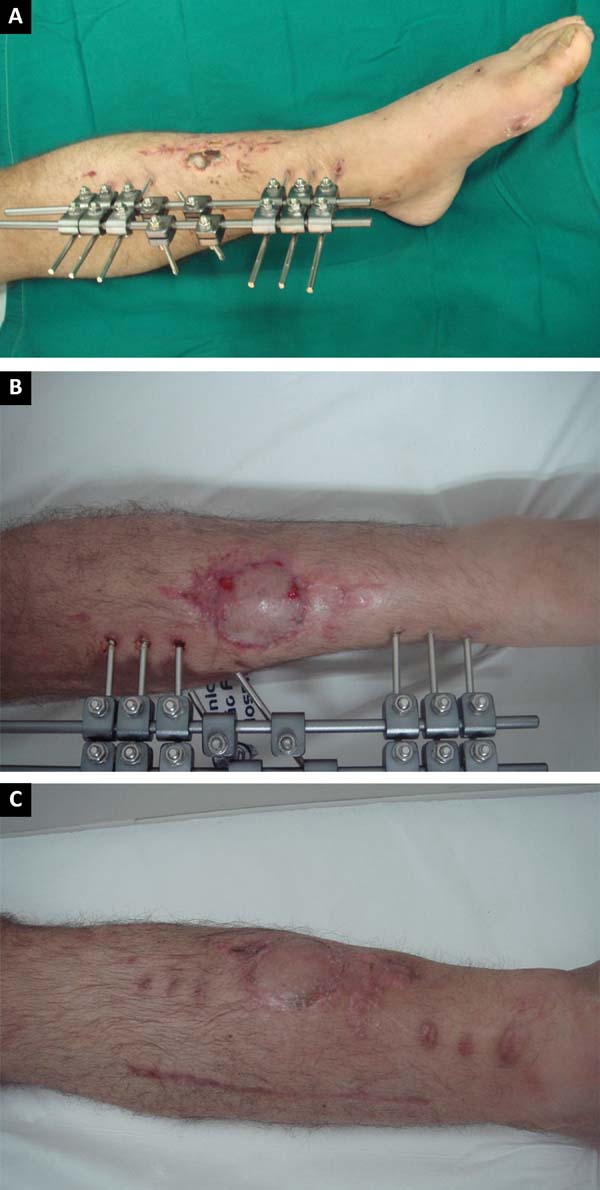
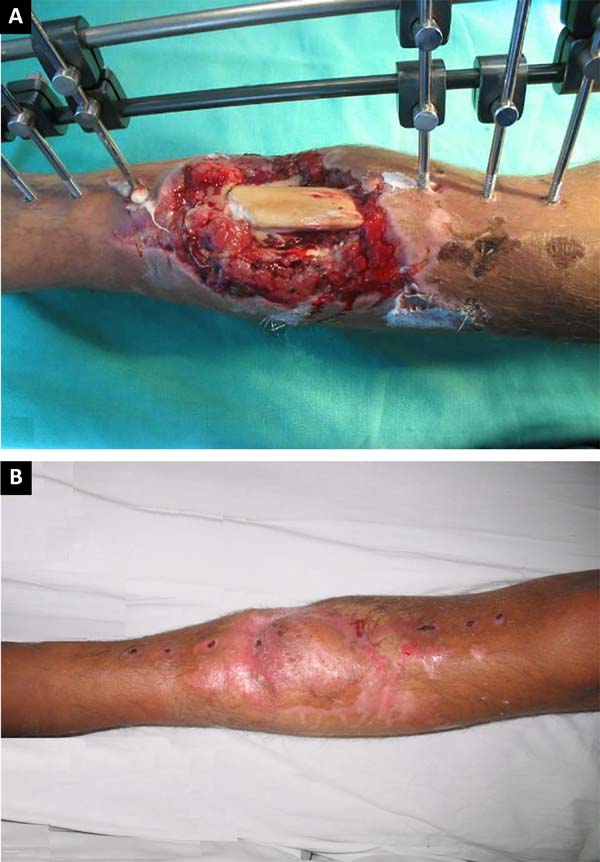
hemisoleus flap was used (Figures 10A,
B, and C) to repair substance losses in the lower distal third of the leg
caused by burns and exposed tibial fractures. The remaining six cases were
treated with proximally pediculated hemisoleus flaps (Figures 11, 12, and
13) for repair of wounds with
mid-tibial bone exposure caused by automobile accidents and burns. The mean age
was 40.5 years (range, 20–68 years). Seven patients (77.7%) underwent surgery
after fracture stabilization with external fixators and had palpable and normal
pulses.
Table 1 - Results.
| Patient |
Age(years) |
Sex |
Injury location |
Injury cause |
Pretreatment |
Medialhemisoleusflap |
Flap complication or skin grafting |
Fracturecomplications |
Follow-uptime |
| PCO |
20 |
Male |
Open fracture, medial 1/3 of the left tibia |
Automobileaccident |
Fasciosubcutaneous reverse calf flap |
Proximalpedicle |
- |
- |
30 months |
| LMF |
27 |
Male |
Open fracture, medial 1/3 of the left tibia |
Automobileaccident |
"Cross-leg" and fracture fixation |
Proximalpedicle |
- |
Pseudo-arthrosis |
27 months |
| APO |
30 |
Male |
Open fracture, medial 1/3 of the right tibia |
Automobileaccident |
Fracture fixation, thoracotomy, and
laparotomy
|
Proximalpedicle |
Donor-site hematoma |
- |
22 months |
| APO |
30 |
Male |
Open fracture, medial 1/3 of the left tibia |
Automobileaccident |
Hemisoleus medial right flap |
Proximalpedicle |
- |
- |
22 months |
| GMS |
41 |
Male |
Medial malleolus right exposure |
Overheated metal burn |
Debridement and dressings |
Reverse |
- |
- |
4 months |
| OMS |
54 |
Male |
Open fracture, distal 1/3 of the left tibia |
Home accident (fall) |
Fracture fixation |
Reverse |
Partial flap loss |
- |
18 months |
| MEF |
68 |
Female |
Open fracture, distal 1/3 of the right tibia |
Home accident (fall) |
Fracture fixation |
Reverse |
Partial skin graft loss |
Chronicosteomyelitis |
12 months |
| JASB |
32 |
Male |
Open fracture, medial 1/3 of the left tibia |
Automobileaccident |
Fracture fixation |
Proximalpedicle |
Partial skin graft loss |
- |
6 months |
| MLRD |
63 |
Female |
Open fracture, medial 1/3 of the right tibia |
Electrical burn |
Debridement and dressings |
Proximalpedicle |
Partial skin graft loss |
- |
5 months |
Figure 10 - A: Exposed fracture of one third of the distal
tibia; B: Medial reverse flap: final aspect of the
donor area of the flap and the recipient area with a skin graft;
C: Results at 6 months postoperative.
Figure 10 - A: Exposed fracture of one third of the distal
tibia; B: Medial reverse flap: final aspect of the
donor area of the flap and the recipient area with a skin graft;
C: Results at 6 months postoperative.
Figure 11 - A: Preoperative view of proximal pedicle flap;
B: Results at 30 days postoperative;
C: Results at 6 months postoperative.
Figure 11 - A: Preoperative view of proximal pedicle flap;
B: Results at 30 days postoperative;
C: Results at 6 months postoperative.
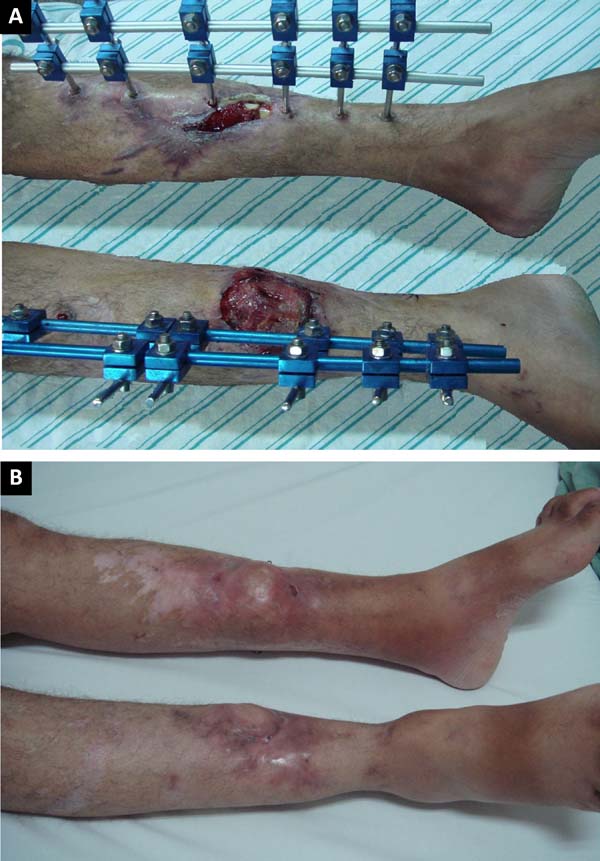
Figure 12 - A: Right leg with created flap and left leg with
exposed tibial facture; B: Results at 24 months
postoperative.
Figure 12 - A: Right leg with created flap and left leg with
exposed tibial facture; B: Results at 24 months
postoperative.
Figure 13 - A: Preoperative view of proximal pedicle flap;
B: Results at 36 months postoperative.
Figure 13 - A: Preoperative view of proximal pedicle flap;
B: Results at 36 months postoperative.
Two patients (22.2%) previously underwent rotation of other flaps with
unsatisfactory results. The first one developed complete necrosis of the
fasciosubcutaneous reverse flap of the calf caused by the large wound extension
and prolonged bone exposure (chronic osteomyelitis). The other patient did not
tolerate the immobilization in the postoperative period of a “cross leg.”
One of the patients (11.1%) presented bleeding in the donor area of the flap on
the 10th postoperative day caused by disruption of the sectioned
distal pedicle that was treated with immediate drainage and there was no
compromise of the flap vascularization.
Partial necrosis of the medial reverse hemisoleus flap occurred in one patient
(11.1%); the probable causes are related to the presence of local infection,
severe venous congestion in the postoperative period, compression by the
fasciocutaneous tunnel, and advanced patient age. In this case, the sequential
therapy was conservative (debridement, treatment of osteomyelitis and assisted
dressings under vacuum) and wound healing was achieved.
Partial-thickness skin grafts were performed at the same surgical time in eight
of the nine cases; in three patients (33.3%), there were insignificant losses
of
skin graft integration without the need for additional surgical procedures
(Figure 11B).
The mean surgical time was 2 hours, while the hospital stay after the flap
production was 4–28 days (mean, 17.7 days). All patients achieved complete wound
healing. In the postoperative follow-up period, three patients required
orthopedic surgical treatment with grafts and bone expansion to correct
pseudoarthrosis. The minimum postoperative follow-up was 4 months and the mean
follow-up time was 16.2 months.
DISCUSSION
Therapeutic options for repairing complex wounds of the medial and distal thirds
of the leg cannot yet be considered consensual. Several flaps have been
described; however, the muscular flaps, particularly the hemisoleus flap,
require intermediate procedures between the fasciocutaneous flaps and transfer
free flaps.
All patients in the series underwent medial hemisoleus flap transfer based on the
favorable location of the wound, and the main feature of this flap is
maintaining plantar flexion at the ankle joint. The soleus muscle is
responsible, together with the gastrocnemius muscle, for stabilizing the leg
over the foot, that is, for maintaining posture and preventing the body from
falling forward when in the upright position21. Preservation of the lateral belly of the soleus at the donor
area reduces the use of compensatory mechanisms that arise when the soleus is
fully rotated: short step, reduced ability to tilt the body forward, and
precocious contralateral calcaneus wound13.
Associated with this precious advantage, hemisoleus flaps are interesting because
they promote low morbidity in the donor bed with the need for skin grafts in
these areas. This was confirmed in the series of patients in this study, in
which all donor areas were closed primarily without epidermolysis or necrosis.
Only one patient had late complications (bleeding) in the donor area and no
impairment of the final healing result in the flap donor and recipient
areas.
The concept that covering wounds with a rich vascular supply is important in
cases of bone exposure favoring fracture consolidation is widely advocated by
adherents of muscle flaps1,6,11,13,19. However,
controversy persists regarding the true beneficial potential of the rich
vascularization of muscle flaps in primary fracture consolidation3, which seems to have been suggested in
this series in which three patients (37.5%) still required orthopedic surgical
complements for fracture healing (Table 1). On the other hand, this rich vascularization is certainly responsible
for the high integration rates of the skin graft on the muscle, a fact not
observed in the use of fascial and fasciocutaneous flaps2,7,19, where the
capacity to supply adequate coverage thickness and well vascularized continues
to be questioned.
The mean surgical time did not exceed the 2 hours, a fact that has a direct
positive impact on technical execution, treatment cost, and postoperative
morbidity rate.
The importance of microsurgical transfer free flaps is based on the potential of
promoting coverage in a single procedure with tissue considered healthy and not
associated with trauma. It combines the development of vascular techniques,
surgical microscopes, delicate instruments, microsurgical threads, and
differentiated surgical strategies that, in most cases, are the major limiting
factors of this therapy9,14,16. In this context, the hemisoleus flap can be very useful
in the treatment of substance losses of the medial and lower leg when the
compromised area is <50 cm². Conventional soleus muscle flaps may cover a
mean area of 26 cm² according to Hughes et al. 4, who performed numerical studies on cadavers comparing the arcs of
rotation of different muscle flaps of the leg.
Pu and Dumanian stated that wounds up to 50 cm² can be safely repaired with
medial hemisoleus flaps, reporting a significant gain in extent for this
flap1,6.
Detailed knowledge of the morphological and neurovascular anatomy of the soleus
muscle is obviously the initial condition for performing this procedure. For
this purpose, previous dissections in cadavers can be very enlightening and
illustrative22. The technical
refinements described by Pu involve the delicacy of the surgical approach to
the
muscle, dissection of the main pedicles with the purpose of lengthening the
rotation arc, use of sharp blades for the medial soleus tendon transversal
section that is intimately connected to the gastrocnemius tendon, and suture
of
the medial tendon of the hemisoleus in the lateral segment of the muscle to
minimize functional losses16,20.
By combining this knowledge with the described angiosome principles, especially
for reverse flaps, high success rates can be achieved22. The main complications described (postoperative
bleeding, partial graft losses, and failure of previously used flaps) were
observed at the beginning of the series; therefore, they may be related to
technique and indications. The use of the more accurate technique and more
rigorous indication criteria, which occurred gradually throughout the series,
reduced the complication rates of this flap. Therefore, the learning curve is
a
considerable factor in the improvement of this surgical technique.
Some complementary steps are also being gradually added in the treatment of these
wounds, such as: assisted vacuum closure in the preoperative preparation to
reduce injury extent, preoperative angiography in the evaluation of the flap’s
vascular potential, postoperative Doppler use, design of the most viable flap
design, and description of patch associations for extensive wound closure1,6,13,23. The
insertion of these concepts into the therapeutic plan may reduce the
postoperative complication rates and increase the technical reliability of this
procedure.
CONCLUSIONS
The use of medial hemisoleus proximal (direct flow) or distal (reverse flow)
flaps is very useful for repairing substance losses from the medial and distal
thirds of the leg and allow wound coverage with intermediate thickness tissues,
rich local vascularization, a low donor-area morbidity index, preservation of
the plantar motor function, faster postoperative rehabilitation, accessible
surgical technique, and shorter operative time.
These flaps may be considered a secondary alternative to transfer free flaps in
the reconstruction of these defects or even the first treatment option in cases
in which a minor injury or the presence of comorbidities does not justify the
complexity of using a transfer free flap.
The association with the angiosome concept and the technical refinements
described recently increased hemisoleus flap manufacture safety, reaffirming
that the establishment and observation of the indication criteria in
preoperative planning is essential to guarantee greater therapeutic success and
reduce the postoperative complication rate.
COLLABORATIONS
|
EJC
|
Analysis and/or data interpretation, conception and design study,
conceptualization, final manuscript approval, realization of
operations and/or trials, supervision, writing - review &
editing.
|
|
MLPN
|
Analysis and/or data interpretation, data curation, final manuscript
approval, realization of operations and/or trials, software, writing
- original draft preparation.
|
|
LACF
|
Analysis and/or data interpretation, data curation, final manuscript
approval, writing - original draft preparation.
|
|
DMCJ
|
Analysis and/or data interpretation, data curation, final manuscript
approval, realization of operations and/or trials, writing -
original draft preparation.
|
REFERENCES
1. Schierle CF, Rawlani V, Galiano RD, Kim JY, Dumanian GA. Improving
outcomes of the distally based hemisoleus flap: principles of angiosomes in flap
design. Plast Reconstr Surg. 2009;123(6):1748-54. DOI:
10.1097/PRS.0b013e3181a65a74.
2. Verhelle N, Vranckx J, Van den Hof B, Heymans O. Bone exposure in
the leg: is a free muscle flap mandatory? Plast Reconstr Surg.
2005;116(1):170-7.
3. Braga-Silva J, Martins PDE, Roman JA, Gehlen D. Utilização do
retalho adipofascial reverso nas perdas de substância cutânea do terço distal
da
perna e pé. Rev Soc Bras Cir Plást. 2005;20(3):182-6.
4. Hughes LA, Mahoney JL. Anatomic basis of local muscle flaps in the
distal third of the leg. Plast Reconstr Surg.
1993;92(6):1144-54.
5. Belém LFMM, Lima JCSA, Ferreira FPMF, Ferreira EM, Penna FV, Alves
MB. Retalho sural de fluxo reverso em ilha. Rev Soc Bras Cir Plást.
2007;22(4):195-201.
6. Pu LLQ. Further experience with the medial hemisoleus muscle flap
for soft-tissue coverage of a tibial wound in the distal third of the leg. Plast
Reconstr Surg. 2008;121(6):2024-8. DOI
10.1097/PRS.0b013e318171240c
7. Canton EJ, Barbosa LS, Ferreira AB, Conde CMY, Respeita EMZ,
Gonçalves LB, et al. Reconstrução do terço inferior da perna com retalho
fasciocutâneo de pedículo distal. HU Rev. 2002;28:364-6.
8. Martins GB, Moreira AL, Viana FO. Reconstrução de lesões de partes
moles do calcanhar com o uso de retalhos fasciocutâneos. Rev Bras Cir Plást.
2009;24(1):104-9.
9. Batista J. Retalho supramaleolar de fluxo reverso: aplicações
clínicas. Rev Bras Cir Plást. 2011;26(1):140-6.
10. Cortez M, Borges LG, Lima SCA. Um novo retalho muscular para
cobertura do terço inferior da perna e do pé. Rev Bras Ortop.
1993;28(9):687-93.
11. Bacelar TH. Utilização do músculo sóleo para perdas musculocutâneas
de terço médio da perna. Rev Bras Cir Plást. 2011;26(2):211-20.
12. Magee WP Jr, Gilbert DA, McInnis WD. Extended muscle and
musculocutaneous flaps. Clin Plast Surg. 1980;7(1):57-70.
13. Tobin GR. Hemisoleus and reversed hemisoleus flaps. Plast Reconstr
Surg. 1985;76(1):87-96.
14. Souza Filho MVP, Teixeira JCEO, Castro OC. Retalho hemisolear
reverso na reconstrução de defeito do terço distal da perna. Rev Bras Cir Plást.
2011;26(4):710-3.
15. Ahmad I, Akhtar S, Rashidi E, Khurram MF. Hemisoleus muscle flap in
the reconstruction of exposed bones in the lower limb. J Wound Care.
2013;22(11):635,638-40, 642. DOI: 10.12968/jowc.2013.22.11.635
16. Pu LLQ. The medial hemisoleus muscle flap for soft-tissue coverage
of an open wound in the distal leg: P74. Plast Reconstr Surg. 2005;116(Suppl
3):215-6.
17. Costa AC. Retalho ântero-lateral da coxa - estudo anatômico em
brasileiros [Tese de doutorado]. São Paulo: Faculdade de Ciências Médicas da
Santa Casa de São Paulo; 2006. 150 p.
18. Taylor GI, Palmer JH. The vascular territories (angiosomes) of the
body: experimental study and clinical applications. Br J Plast Surg.
1987;40(2):113-41.
19. Mathes SJ, Nahai F. Clinical applications for muscle and
musculocutaneous flaps. St Louis: Mosby; 1982.
20. Figueiredo JCA, Freitas AG, Arantes HL. Retalhos musculares e
musculocutâneos. In: Mélega JM, Bastos JAV, Mélega LM, eds. Cirurgia plástica,
fundamentos e arte, princípios gerais. Rio de Janeiro: MEDSi; 2002.
p.121-39.
21. Moore KL. O membro inferior. In: Moore KL. Anatomia Orientada para a
Clínica. 2a ed. Rio de Janeiro: Guanabara; 1985.
22. Bourdais-Sallot A, Pare A, Herard C, Duclert-Bompaire M, Pucheux J,
Terrier LM, et al. Distally Based Medial Hemisoleus Muscle Flap: Anatomic and
Angiographic Study of 18 Lower Limbs. Ann Plast Surg. 2017;79(1):73-8. DOI:
10.1097/SAP.0000000000000997
23. Junior JMC, Maciel LCL, Antonini PA, Bandeira TRS. Retalhos
músculo-cutâneo para tratamento de deformidades de membros inferiores. Rev Bras
Cir Plást. 2008;23(Supl):95.
1. Hospital Ibiapaba Cebams, Barbacena, MG,
Brazil.
2. Sociedade Brasileira de Cirurgia Plástica, São
Paulo, SP, Brazil.
3. Faculdade de Ciências Médicas e da Saúde,
Suprema, Juiz de Fora, MG, Brazil.
4. Faculdade de Medicina de Barbacena, Barbacena,
MG, Brazil.
5. Faculdade de Medicina, Universidade Federal de
Viçosa, Viçosa, MG, Brazil.
Corresponding author: Emiliano José Canton, Rua Padre Anchieta, nº
48, sala 307 - Centro - Barbacena, MG, Brazil, Zip Code 36200-036. E-mail:
emiliano.canton@hotmail.com
Article received: May 18, 2018.
Article accepted: October 1, 2018.
Conflicts of interest: none.