INTRODUCTION
Breast cancer is currently one of the most common health problems in the world.
In Brazil, its incidence has been increasing gradually. Excluding skin cancer,
breast cancer is the most frequent type of cancer that affects women
worldwide1.
Total mastectomy, especially in some developing countries and centers further
away, is still widely employed for the treatment of breast cancer. This surgery
and other adjuvant therapies may contribute to the development of physical and
psychological complications, which can negatively influence patients’ quality of
life2-4. After mastectomy, the loss of breast alters the body
image of women and yields a feeling of mutilation and loss of femininity and
sensuality5,6.
In an attempt to reduce the negative feelings related to the disease and its
treatment, improve self-esteem, and address the loss of breast, many women opt
for surgical reconstruction7. This is a
safe procedure, which does not increase the risk of recurrence, interfere with
detection of the disease, or lead to delay in adjuvant therapies. There are
several surgical procedures such as conservative techniques, adjacent flaps,
alloplastic materials, and myocutaneous pedicle flaps microcirúrgicos8-12.
Law 12, 802/2013 requires the Unified Health System (SUS) to provide
reconstructive plastic surgery of the breast soon after mastectomy when clinical
conditions permit. However, there is often no structure in public hospitals to
perform such procedures. Further, there are deficiencies ranging from lack of
operating rooms to the absence of qualified medical personnel and suitable
material. Thus, reconstruction is for the second half. However, owing to the
high demand of the SUS, many of these patients are waiting for reconstruction in
rows, which often seem intermináveis12.
The Brazilian Society of Plastic Surgery (SBCP) estimates that the average
waiting time for reconstruction is 10 years; in 2015, only 1100 breast
reconstructions were performed by the SUS12.
Many civil institutions, such as the SBCP, in partnership with the NHS, often
offer solutions to mitigate these situations. Among these solutions, we can
mention Mutirões12.
From October 24 to 29, 2016, the SBCP promoted the 2nd National Task
Force of Breast Reconstruction (NTFBR), which included the participation of more
than 800 professionals in the specialized area. Approximately 840 women who
underwent mastectomy were operated on for free by plastic surgeons, aiming at
the possibility of rebuilding mamária12.
The Plastic Surgery Service of Walter Cantídio University Hospital (SCPMR-HUWC)
also collaborated on this project in 2016, with heterogeneous participation of
plastic surgeons and completion of 16 breast reconstructions.
OBJECTIVE
The objective of this study was to analyze the results of the 2nd
NTFBR, held in October 2016 in SCPMR-HUWC, with a heterogeneous group of plastic
surgeons.
METHODS
A prospective cohort study was conducted, in which 16 patients who underwent
breast reconstruction in SCPMR-HUWC and were included in the second NTFBR held
in October 2016, were evaluated.
The study was approved by the Ethics in Research CAAE: 69439917.0.0000.5045 and
was conducted in accordance with Resolution 466/12 of the National Health
Council, which approved the regulatory guidelines and standards for research
involving humans.
The Task Force in question included all patients who were in the queue for breast
reconstruction surgery in SCPMR-HUWC. We collected the following data: age,
waiting time in the queue, type of breast reconstruction performed, length of
hospital stay, and postoperative complications.
The patients were followed up for 6 months; their data were tabulated and
analyzed by the investigators using the statistical software
Epi-Info®, and were considered significant at p
< 0.05 with a confidence interval of 95%.
RESULTS
A total of 16 female patients were subjected to post-mastectomy breast
reconstruction and cardiovascular and surgical risk evaluations; all patients
were found to be fit for reconstruction.
No patient was under treatment with chemotherapy (QMT) or radiotherapy (RTX). All
patients were to undergo delayed reconstruction (more than 1 year after
mastectomy and free from any adjuvant procedure like QMT and RTX for more than a
year).
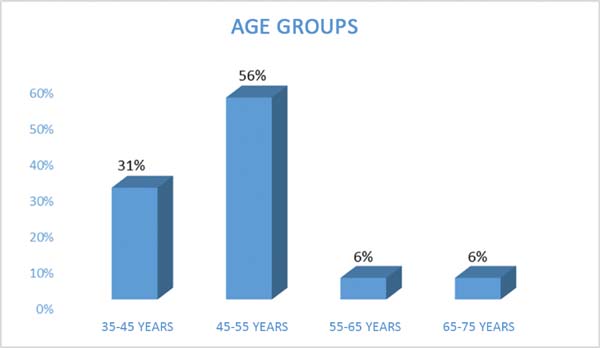
The patients’ ages ranged from 39 to 72 years, mean 49 years for the
reconstruction (Figure 1).
Figura 1 - Age of patients undergoing post-mastectomy breast
reconstruction.
Figura 1 - Age of patients undergoing post-mastectomy breast
reconstruction.
None of the patients presented with skin disorders, radiodermatitis, pyoderma,
tumors, or significant deformities in the surgical site.
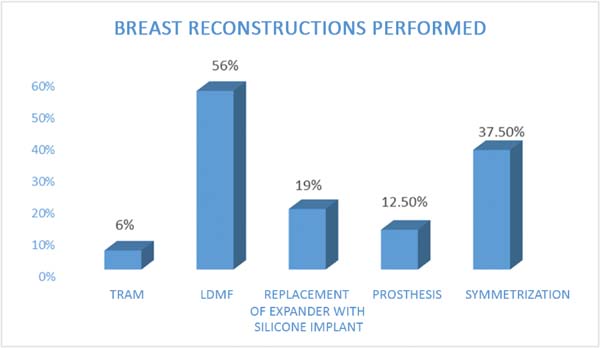
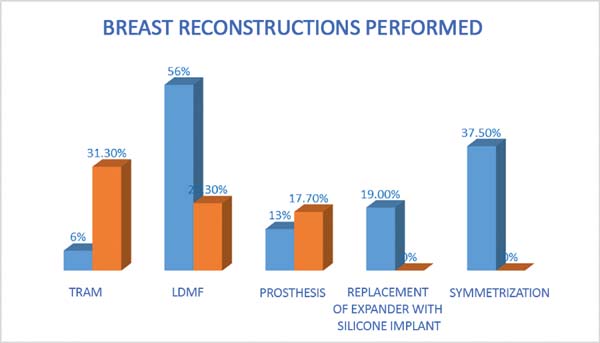
The following types of reconstruction were performed: one (6%) rectus muscle
myocutaneous flap (TRAM) creation, nine (56%) latissimus dorsi muscle
myocutaneous flap (RGD) creations, five prosthesis [three (19%) with exchange
with unilateral prosthesis expanders and two (12.5%) with unilateral prosthesis
(right)] implantations, and six (37.5%) symmetrizations (Figures 2 to 6).
The length of hospital stay ranged from 1 to 5 days; approximately 82% of the
patients passed 4 days or less.
Figure 2 - Number of breast reconstructions performed in the National
Program for Breast Reconstruction according to the technique LDMF,
latissimus dorsi myocutaneous flap; RAMF, rectus abdominis
myocutaneous flap.
Figure 2 - Number of breast reconstructions performed in the National
Program for Breast Reconstruction according to the technique LDMF,
latissimus dorsi myocutaneous flap; RAMF, rectus abdominis
myocutaneous flap.
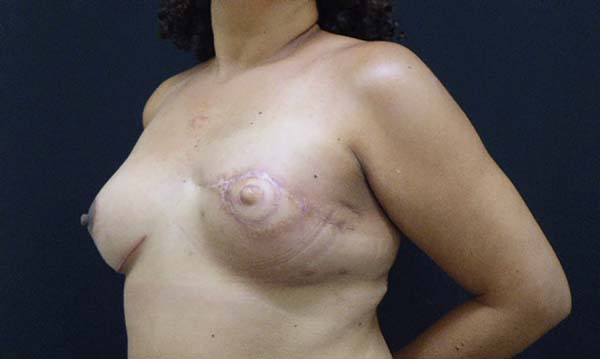
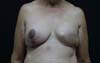
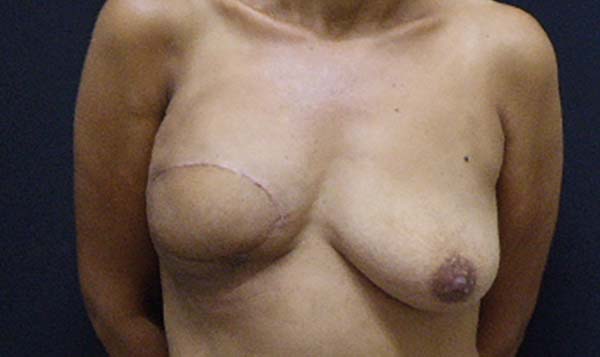
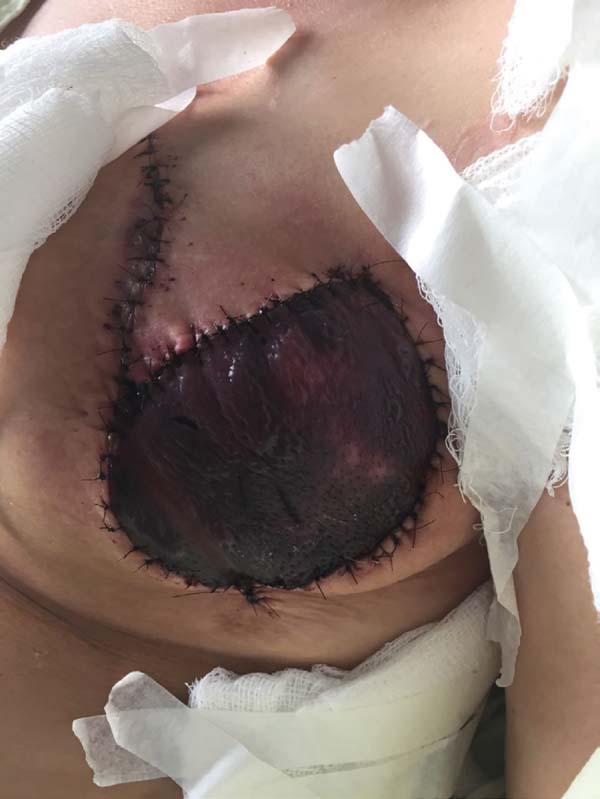
Figura 3 - Breast reconstruction using the latissimus dorsi myocutaneous
flap after mastectomy.
Figura 3 - Breast reconstruction using the latissimus dorsi myocutaneous
flap after mastectomy.
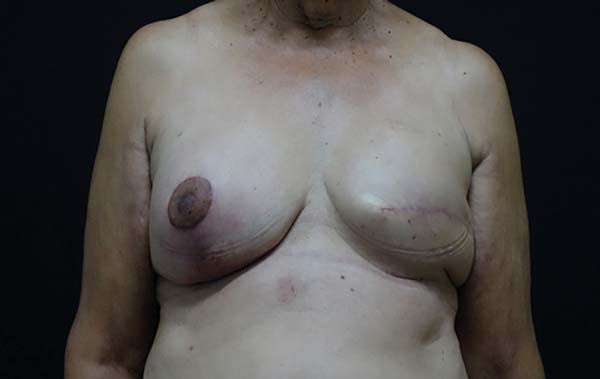
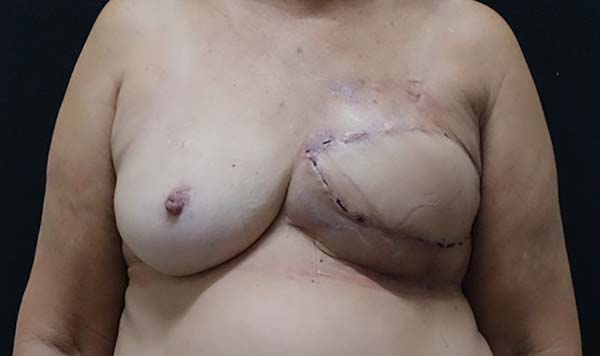
Figure 4 - Breast reconstruction, replacement of expander with silicone
implant and post-mastectomy symmetrization.
Figure 4 - Breast reconstruction, replacement of expander with silicone
implant and post-mastectomy symmetrization.
Figura 5 - Breast reconstruction using the latissimus dorsi myocutaneous
flap after mastectomy.
Figura 5 - Breast reconstruction using the latissimus dorsi myocutaneous
flap after mastectomy.
Figura 6 - Breast reconstruction using the rectus abdominis myocutaneous
flap after mastectomy.
Figura 6 - Breast reconstruction using the rectus abdominis myocutaneous
flap after mastectomy.
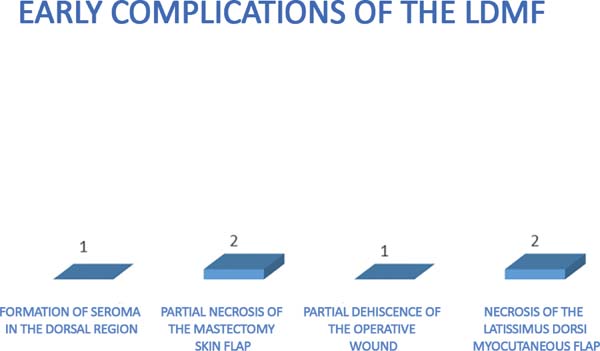
The complications were divided into early (those that occurred within 30 days
after surgery) and late (those that occurred after 30 days). The earliest
complications observed were seroma in the dorsal region (13%), partial necrosis
of the mastectomy skin (6%), dehiscence of the operative wound (13%), and
necrosis of the latissimus dorsi flap (6%) (Figures 7 and 8).
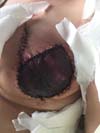
Figura 7 - Early complications of breast reconstruction using the latissimus
dorsi myocutaneous flap after mastectomy.
Figura 7 - Early complications of breast reconstruction using the latissimus
dorsi myocutaneous flap after mastectomy.
Figura 8 - Early complications of breast reconstruction using the latissimus
dorsi myocutaneous flap after mastectomy.
Figura 8 - Early complications of breast reconstruction using the latissimus
dorsi myocutaneous flap after mastectomy.
Figure 9 - Number of breast reconstructions performed according to technique
Plastic Surgery Service of the Walter Cantídio University Hospital
(in blue); statistical analysis of the study by Cosac et al.
16 (in red). LDMF, latissimus
dorsi myocutaneous flap; TRAM, rectus abdominis myocutaneous
flap.
Figure 9 - Number of breast reconstructions performed according to technique
Plastic Surgery Service of the Walter Cantídio University Hospital
(in blue); statistical analysis of the study by Cosac et al.
16 (in red). LDMF, latissimus
dorsi myocutaneous flap; TRAM, rectus abdominis myocutaneous
flap.
None of the risk factors (i.e., hypertension, diabetes mellitus, smoking, BMI,
and age) was significantly associated with the early complications. All early
complications occurred only in the patients with RGDs.
None of the 16 patients had any late complications such as implant coverage
changes, capsular contraction, or muscle and skin atrophies.
DISCUSSION
Breast reconstruction is gaining an increasingly important role in the treatment
of breast cancer because of the proven psychological and physical benefits for
patients. This procedure favors faster return of these patients to social life,
improves immunity, and thus offers better prognosis in the treatment of the
disease13,14.
Many reconstruction techniques have been developed over the years. The most
commonly used procedures are as follows:
Creation of myocutaneous pedicle flaps, such as latissimus dorsi muscle
flaps;
Creation of retail transverse rectus abdominis muscle flaps;
Implantation of alloplastic materials, such as temporary or permanent
tissue expanders;
Implantation of silicone.
In this scenario, there is evidence of indications of breast reconstruction with
the use of local flaps, and the alloplastic materials RGD over the TRAM, which
has a higher morbidity and systemic site15.
In the study by Cosac et al.16, the most
used technique was TRAM reconstruction (31.3%), followed by RGD (30%), and
prosthesis (17.7%). However, reconstruction using exchange expander prosthesis
and symmetrization were not studied. In our study, the type of reconstruction
performed constituted TRAM (6%), RGD (56%), 13% and symmetrization prosthesis
(37.5%) (Figure 9).
For the treatment for breast cancer, adjuvant radiation therapy is often
performed after mastectomy in women diagnosed with breast cancer stages II and
III. This increases local control, disease-free survival, and survival
globally17-20.
Despite the improvement of the oncological results, adjuvant radiation therapy in
women with breast cancer may worsen the aesthetic results due to tissue
atrophies and capsular contractures and increase the risk of loss of rebuilding
mamária21.
Seroma in the donor area of the latissimus dorsi muscle is the most common
complication of the procedure, with a reported rate of 16% to 79%22-25.
However, the significance of seroma as a major complication requiring further
surgery is low. In the present study, we observed a rate of 13% seroma. Gart et
al.15 reported that 1079 patients
from the database of American College of Surgeons National Surgical Improvement
Program (ACS-NSQIP) undergoing RGD, showed early complications like reoperations
(5.7%), cutaneous infections (3.3%), necrosis (1.3%), surgical wound dehiscence
(0.6% ), and other complications (3.2%) 26.
Early complications were observed in this joint Task Force of the case 1 (6%)
partial mastectomy skin necrosis and 2 cases (13%) partial dehiscence of the
surgical wound. These complications occurred probably due to the ineffective
surgical procedure resulting in patchwork, thin mastectomy, and hypoperfused
skin. There was 1 case of necrosis of the flap of latissimus dorsi, but no cases
of infection or other clinical complications.
In a case series of 100 cases, Perdikis et al.22 observed a capsular contracture rate of in patients undergoing
RGD and 6% in those with silicone implants. In another series of 53 cases, Venus
& Prinsloo26 observed 7.4% of
capsular contracture in cases that required capsulotomy and 33% of capsular
contracture in those that did not require surgery.
In the present study of 16 patients, none showed any changes in implant coverage
like muscle and skin atrophy.
We note that five days were enough to clear the queue of the 16 SCPMR-HUWC
patients waiting for breast reconstruction.
The vast majority of patients were discharged in less than four days, which shows
that this kind of a joint Task Force does not hinder the operation of the
hospital hotel structure.
The surgeries were performed on 2 consecutive weekends, and the surgical
operation was also unaffected, because the SCPMR-HUWC elective surgeries are
mostly performed during the week.
Because the task forces comprised of a heterogeneous group of plastic surgeons
from other institutions, it encouraged an exchange of experiences and
innovations in techniques, as well as established new partnerships and
strengthened old ties.
Plastic surgery has an important role in the treatment of patients with breast
cancer. In this work, a high degree of satisfaction in patients was observed due
to the results and few complications. However, in spite of the surgeries being
elective and performed by senior plastic surgeons, we had a high number of
complications. This rate was consistent with that in the literature, probably in
the study in question, of a fortuitous nature. Thus, we conclude that the joint
Task Forces of breast reconstruction for post mastectomy cases are a viable
alternative in terms of public health.
COLLABORATIONS
|
AM
|
Analysis and/or interpretation of data; statistical analyses;
conception and design of the study; completion of surgeries and/or
experiments; writing the manuscript or critical review of its
contents.
|
|
SGPP
|
Analysis and/or interpretation of data; final approval of the
manuscript; conception and design of the study; completion of
surgeries and/or experiments; writing the manuscript or critical
review of its contents.
|
REFERENCES
1. Brasil. Ministério da Saúde. Instituto Nacional de Câncer. José
Alencar Gomes da Silva (INCA). Estimativa 2012: Incidência de Câncer no Brasil.
Rio de Janeiro: INCA; 2011.
2. Cheville AL, Tchou J. Barriers to rehabilitation following surgery
for primary breast cancer. J Surg Oncol. 2007;95(5):409-18. DOI: http://dx.doi.org/10.1002/jso.20782
3. Ribeiro RVE, Silva GB, Augusto FV. Prevalência do transtorno
dismórfico corporal em pacientes candidatos e/ou submetidos a procedimentos
estéticos na especialidade da cirurgia plástica: uma revisão sistemática com
meta-análise. Rev Bras Cir Plást. 2017;32(3):428-35.
4. Ching AW, Costa MP, Brasolin AG, Ferreira LM. Influência das
complicações pós-operatórias no insucesso da reconstrução de mama imediata com
implante de silicone. Rev Bras Cir Plást. 2015;30(2):182-9.
5. Alves VL, Sabino Neto M, Abla LEF, Oliveira CJR, Ferreira LM.
Avaliação precoce da qualidade de vida e autoestima de pacientes mastectomizadas
submetidas ou não à reconstrução mamária. Rev Bras Cir Plást.
2017;32(2):208-17.
6. Sheppard LA, Ely S. Breast cancer and sexuality. Breast J.
2008;14(2):176-81. DOI: http://dx.doi.org/10.1111/j.1524-4741.2007.00550.x
7. Keith DJ, Walker MB, Walker LG, Heys SD, Sarkar TK, Hutcheon AW, et
al. Women who wish breast reconstruction: characteristics, fears, and hopes.
Plast Reconstr Surg. 2003;111(3):1051-6. DOI: http://dx.doi.org/10.1097/01.PRS.0000046247.56810.40
8. Cammarota MC, Galdino MCA, Lima RQ, Almeida CM, Ribeiro Junior I,
Moura LG, et al. Avaliação das simetrizações imediatas em reconstrução de mama.
Rev Bras Cir Plást. 2017;32(1):56-63.
9. Oliveira LD. Análise retrospectiva da casuística pessoal em
mamoplastia redutora utilizando a técnica de pedículo inferior em suas
diferentes indicações. Rev Bras Cir Plást. 2016;31(3):321-7.
10. Miranda RE, Pereira JB, Gragnani Filho A. Uso de duas telas de
polipropileno na área doadora do TRAM para reconstrução mamária: avaliação na
incidência de hérnia e abaulamento abdominal. Rev Bras Cir Plást.
2015;30(4):560-6.
11. Tavares-Filho JM, Franco D, Moreto L, Porchat C, Franco T.
Utilização do retalho miocutâneo de grande dorsal, com extensão adiposa, nas
reconstruções mamárias: uma opção para preenchimento do polo superior. Rev Bras
Cir Plást. 2015;30(3):423-8.
12. Saldanha Filho OR, Saldanha O, Cação E, Menegazzo MR, Cazeto D,
Canchica AC, et al. Reconstrução de mama com miniabdominoplastia reversa. Rev
Bras Cir Plást. 2017;32(4):505-12.
13. Sociedade Brasileira de Cirurgia Plástica (SBCP). Câncer de mama:
mutirão de reconstrução mamária vai realizar 842 cirurgias. São Paulo; 2016.
[acesso 2017 Abr 16]. Disponível em: http://www2.cirurgiaplastica.org.br/2016/10/20/cancer-de-mama-mutirao-de-reconstrucao-mamaria-vai-realizar-842-cirurgias/
14. Veiga DF, Veiga-Filho J, Ribeiro LM, Archangelo I Jr, Balbino PF,
Caetano LV, et al. Quality-of-life and self-esteem outcomes after oncoplastic
breast-conserving surgery. Plast Reconstr Surg. 2010;125(3):811-7. PMID:
20195109 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181ccdac5
15. Bellino S, Fenocchio M, Zizza M, Rocca G, Bogetti P, Bogetto F.
Quality of life of patients who undergo breast reconstruction after mastectomy:
effects of personality characteristics. Plast Reconstr Surg. 2011;127(1):10-7
PMID: 21200194 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181f956c0
16. Gart MS, Smetona JT, Hanwright PJ, Fine NA, Bethke KP, Khan SA, et
al. Autologous options for postmastectomy breast reconstruction: a comparison of
outcomes based on the American College of Surgeons National Surgical Quality
Improvement Program. J Am Coll Surg. 2013;216(2):229-38. DOI: http://dx.doi.org/10.1016/j.jamcollsurg.2012.11.003
17. Cosac OM, Camara Filho JPP, Barros APGSH, Borgatto MS, Esteves BP,
Curado DMDC, et al. Reconstruções mamárias: estudo retrospectivo de 10 anos. Rev
Bras Cir Plást. 2013;28(1):59-64. DOI: http://dx.doi.org/10.1590/S1983-51752013000100011
18. Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et
al. Postoperative radiotherapy in high-risk premenopausal women with breast
cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group
82b Trial. N Engl J Med. 1997;337(14):949-55. DOI: 10.1056/NEJM199710023371401
DOI: http://dx.doi.org/10.1056/NEJM199710023371401
19. Saliba GAM, Carvalho EES, Silva Filho AF, Alves JCRR, Tavares MV,
Costa SM, et al. Reconstrução mamária: análise de novas tendências e suas
complicações maiores. Rev Bras Cir Plást. 2013;28(4):619-26.
20. Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE,
et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal
women with breast cancer. N Engl J Med. 1997;337(14):956-62. DOI:
10.1056/NEJM199710023371402 DOI:
http://dx.doi.org/10.1056/NEJM199710023371402
21. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et
al.; Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of
radiotherapy and of differences in the extent of surgery for early breast cancer
on local recurrence and 15-year survival: an overview of the randomised trials.
Lancet. 2005;366(9503):2087-106. DOI: http://dx.doi.org/10.1016/S0140-6736(05)67887-7
22. Shah C, Kundu N, Arthur D, Vicini F. Radiation therapy following
postmastectomy reconstruction: a systematic review. Ann Surg Oncol.
2013;20(4):1313-22. DOI: 10.1245/s10434-012-2689-4 DOI: http://dx.doi.org/10.1245/s10434-012-2689-4
23. Perdikis G, Koonce S, Collis G, Eck D. Latissimus dorsi myocutaneous
flap for breast reconstruction: bad rap or good flap? Eplasty. 2011;11:e39.
PMID: 22031843
24. Farah AB, Nahas FX, Mendes JA. Reconstrução mamária em dois estágios
com expansores de tecido e implantes de silicone. Rev Bras Cir Plást.
2015;30(2):172-81.
25. Delay E, Gounot N, Bouillot A, Zlatoff P, Rivoire M. Autologous
latissimus breast reconstruction: a 3-year clinical experience with 100
patients. Plast Reconstr Surg. 1998;102(5):1461-78. DOI: http://dx.doi.org/10.1097/00006534-199810000-00020 DOI:
http://dx.doi.org/10.1097/00006534-199810000-00020
26. Bonomi S, Settembrini F, Salval A, Gregorelli C, Musumarra G,
Rapisarda V. Current indications for and comparative analysis of three different
types of latissimus dorsi flaps. Aesthet Surg J. 2012;32(3):294-302. DOI:
10.1177/1090820X12437783 DOI: http://dx.doi.org/10.1177/1090820X12437783
27. Venus MR, Prinsloo DJ. Immediate breast reconstruction with
latissimus dorsi flap and implant: audit of outcomes and patient satisfaction
survey. J Plast Reconstr Aesthet Surg. 2010;63(1):101-5. DOI:
10.1016/j.bjps.2008.08.064 DOI: http://dx.doi.org/10.1016/j.bjps.2008.08.064
1. University of Belgrade, Belgrade,
Serbia.
2. Hospital Universitário Walter Cantídio,
Universidade Federal do Ceará, Fortaleza, CE, Brazil.
Corresponding author: Aleksandra
Markovic, Av. Beira Mar, 4260 - Praia de Mucuripe - Fortaleza, CE,
Brazil. Zip Code 60165-121. E-mail:
19quepasa19@gmail.com
Article received: September 27, 2017.
Article accepted: June 22, 2018.
Conflicts of interest: none.