ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Mammoplasty: fascioadenocutaneous areolar flap
Mamoplastia: retalho fascioadenocutaneo areolado
ABSTRACT
INTRODUCTION: Several mammoplasty techniques have been described. However, none involves breast resection with preservation of the areola through an oblique multiple pedicle that involves the medial superior neurovascular system and the lateroinferior neurovascular system, or preserves the original areola-glandular unit in the remaining gland. This technique is, in theory, ideal for the treatment of juvenile gigantomastia in nulliparous women who can still benefit from a more functional breast with its primary innervation and the patient's ability to breast-feed preserved. This flap can also present higher safety against ischemia owing to a broad vascular pedicle.
OBJECTIVE: To describe this mammoplasty technique and report the initial series of operated cases.
METHOD: The fascioadenocutaneous areolar flap technique is described in 40 operated breasts, including the quantity of the resected tissue, pre- and postsurgery sensitivity, and complications encountered.
RESULTS: All cases had a satisfactory evolution, without necrosis and with preserved sensitivity of the nipple-areola complex in >70% of the breasts operated.
CONCLUSION: The innervated fascioadenocutaneous areolar flap was safe, functional, and versatile.
Keywords:
Reductive mammoplasty; Areolar flap; Selective mammoplasty.
RESUMO
INTRODUÇÃO: Várias são as técnicas descritas para mamoplastia. Contudo nenhuma contempla a ressecção mamaria com a preservação areolar por meio de um pedículo obliquo múltiplo que envolve o sistema neurovascular superior medial e o sistema neurovascular inferior lateral; alem de preservar na glândula remanescente a unidade areolo-glandular original. Esta técnica mostra se, em tese, ideal para o tratamento da gigantomastia juvenil em nulipara, que ainda pode se beneficiar de uma mama mais funcional e preservada na sua inervação primaria e na sua capacidade de amamentação. Este retalho também pode usufruir de grande segurança contra isquemia em função de um largo pedículo vascular.
OBJETIVO: Descrever a técnica de mamoplastia e relatar a serie inicial de casos operados nesta sistematização.
MÉTODO: Em 40 mamas operadas descreve se a técnica do retalho fascioadenocutaneo areolado com mensuração da quantidade ressecada, sensibilidade pré- e pós- operatório e complicações.
RESULTADOS: Todos os casos tiveram evolução satisfatória, sem necrose e com sensibilidade do complexo areolopapilar (CAP) preservada em mais de 70% das mamas operadas.
CONCLUSÃO: O retalho fascioadenocutaneo areolado inervado mostrou-se seguro, funcional e versátil.
Palavras-chave:
Mamoplastia redutora; Retalho areolado; Mamoplastia seletiva.
INTRODUCTION
Several mammoplasty techniques have been described. Mammoplasty is a procedure designed to improve the shape, volume, and symmetry of the breasts with the least conspicuous scar possible1-3. Preserving the sensitivity and vascularization of the nipple-areola complex (NAC) should also be taken into account. Necrosis is one of the most unwanted complications. The preservation of the areola-glandular unit for its role in breast-feeding is also important, especially in young patients. Ensuring the preservation of sensitivity and breast-feeding ability decreases the morbidity of mammoplasty.
In 1997, the location of the neurovascular structures of the breast was described accurately for the first time to be within a network of ligamentous suspension of the gland attached to the pectoral fascia. In this ligamentous suspension system, a horizontal fibrous septum was described that leads the main vessels and nerves of the NAC from the fifth intercostal space. This description extends the horizon of mammoplasty and has great potential clinical application4.
In general plastic surgery practice, there are two main schools in mammoplasty concerning the NAC: the mono- or bipedicled dermal flap group (Skoog, Strombeck, McKissock, Pitanguy, Arie), based on dermal and subdermal irrigation, and the central and/or inferior pedicled areolar flap group, based on submammary and inframammary chest wall irrigation5-12.
The dermal NAC flap group has great variation and versatility. It involves the removal of the caudal portion of the breast, and allows a large detachment and mobilization of the breast tissue. However, it dissociates the NAC from the rest of the gland and disregards the main irrigation and innervation system of the breast, which would be the deep system originating from the fifth intercostal space. It presents necrosis of the NAC as a major complication, especially in very long flaps of very flaccid or hypertrophic breasts13.
The central and inferior pedicle flap is also very versatile and safe; however, it dissociates the NAC from the superficial subcutaneous neurovascular system, derived from the branches of the anterior intercostal nerves, and does not allow large detachment of the base of the areolar flap. It frequently leads to increased pressure on the vertical scar, an excess of tissue in the bottom pole of the operated breast (pseudoptosis), and an empty upper pole. It is more difficult to use in very amorphous breasts and with large ptosis. The consensus among different authors is to leave the flap with a broad base attached to the chest wall and to the inframammary subcutaneous tissue, which, however, may limit the mobilization of breast tissue2.
Every effort must be employed to increase the safety and the blood supply of the NAC flap. Modifications of traditional techniques are described with the aim to prevent the free NAC flap, which is indicated for large mammary hypertrophy14,15. Recently, the term "selective mammoplasty" has been introduced16,17. With this technique, the combination of a dermal flap with an inferior or central pedicled flap based on the anatomical studies of Würinger has been conceived. This combination, not yet described, is of the superficial and deep neurovascular systems through a single oblique fascioadenocutaneous flap, lateral to the margin of the pectoral muscles with large medial subfascial displacement of the breast.
The objective of this study is to describe the dissection of the fascioadenocutaneous areolar flap for mammoplasty. The technique is based on the combination of already existing concepts, such as the horizontal fibrous breast septum, dermal pedicle, mammary dissection in the subfascial plane, decoupling of breast skin from the underlying gland, and preservation of the areola-glandular unit and of the two superficial and deep neurovascular systems. We also report the beginning of a series of cases corresponding to the clinical application of this technical variation, with the philosophy of preserving breast function as much as possible (sensitivity/breast-feeding), as well as presenting safety and versatility.
METHODS
From August 2009 to May 2013, 20 patients; between 15 and 58 years old (mean age, 30.7 years); with height between 153 and 166 cm and body mass index (BMI) between 21 and 29.7 (mean, 23.95); and with complaints of ptosis, hypertrophy, and/or mammary asymmetry underwent mammoplasty with the fascioadenocutaneous areolar flap.
We recorded the following distances: suprasternal notch-nipple and nipple-inframammary crease. The sum of these distances in each breast and the differences between the distances before and after the surgery (90 days) were calculated. The amount of resected breast tissue and the subjective reports of patients about the perception of changes in the sensitivity of the CAP within a week after the surgery were also recorded. The exclusion criteria were BMI >30 and having stopped breast-feeding <6 months ago. Concerning mammary ptosis, a sum of distances of <29 cm was defined as the absence of ptosis. An upper limit of mammary ptosis for contraindication of this flap was not established.
Breast incision was done according to previous markings, leaving a distance of 20-21 cm from the suprasternal notch to the nipple. Periareolar decortication (Schwarzmann maneuver) was performed. The skin was incised on markings with "Wise" methylene blue, with resection of skin, according to excess tissue, in order to establish the final vertical scar from 5 to 6 cm to the inframammary crease. The mammary gland was dissected in the subfascial plane until the middle breast line, observing the lateral edge of the greater pectoral muscle. The direct perforating arteries of the greater pectoral muscle and the perforating inframammary arteries (caudal to the inframammary incision) were annulled completely. From this point, the detached breast was suspended and the inferior pole was divided through an oblique incision, from the lateral limit of the subfascial dissection in the pectoral muscle, along the edge of the medial pillar up to the height of the NAC. Resection in "wedge" of the lateral margin and upper portion of the mammary gland was done, respecting the deep fascia of the chest wall and decoupling the lateral skin of the breast from the remainder of the mammary gland to the middle breast line marked on the cranial limit of decorticated skin (see Figure 1). Mobilization of monobloc breast tissue (fascioadenocutaneous areolar flap) with medial and cranial advancement was performed to fill the upper pole of the breast. By using tweezers, the new breast "cone" was simulated. Tangential resection of excess caudal and medial tissue was done. Revision of hemostasis and suture of the NAC and of the skin, with a final inverted "T" scar was completed without placement of drains. The flap remained with a broad neurovascular pedicle in its lateral inferoposterior portion (deep lateral intercostal branches) with a medial superior pedicle of the superficial system (superficial anterior intercostal branches) (see Figure 1).
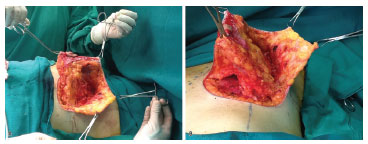
Figure 1. (A) Fascioadenocutaneous flap, with resection of the superior lateral quadrant (side view). (B) Fascioadenocutaneous flap, with resection of the inferior pole and subfascial detachment (caudal view).
RESULTS
In the 40 breasts operated, the suprasternal notch-nipple distance ranged between 23 and 33 cm (mean, 26.8 cm); the nipple-inframammary crease distance ranged between 10 and 14 cm (mean, 12.15 cm); the sum of the two distances ranged between 35 and 47 cm (mean, 38.96 cm); and the difference between the sums before and after surgery ranged between 3.5 and 12.5 cm (mean, 8.74 cm). The amount of resected breast tissue ranged between 58 and 2350 g (mean, 515.57 g) (see Figure 2)
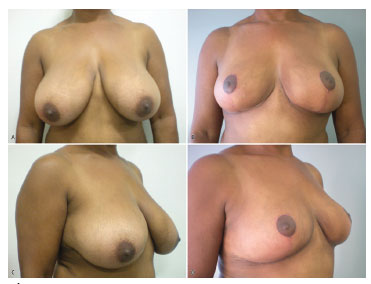
Figure 2. Preoperative and postoperative images.
All patients expressed satisfaction with the surgery, including the shape, volume, and symmetry of the breasts. Of all breasts operated (40), 12 (30%) cases of subjective change/lack of sensitivity of the NAC were reported in the first week after surgery. In the other 28 cases, the patients denied any subjective change in sensitivity. The patient with the largest tissue resection (2350 g) reported an increase in the sensitivity of the breasts and contraction of the CAP. There was no case of NAC loss, hematoma, or infection in the surgical site. There was one case of suffering of part of the areola in one breast (right side) with the nipple 50 cm away from the suprasternal notch. There were no systemic complications or prolongation of hospital stay due to any complications after the surgery.
At late follow-up, hypertrophic healing was observed in four breasts. Approximately 50% of the operated breasts (20) presented pseudoptosis (nipple-inframammary fold distance >8 cm) (mean, 9.8 cm) at the expense of cranial advancement of the inframammary scar. Thus far, none of the patients except for one requested for revision or resection of the skin for treatment of pseudoptosis (see Figure 3).
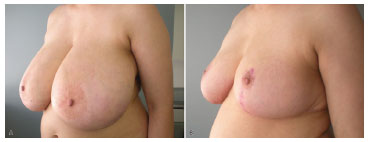
Figure 3. Preoperative and postoperative images.
DISCUSSION
This work describes a technical variation in selective mammoplasty, the use of a fascioadenocutaneous areolar flap, with potential applications in various clinical situations, including reductive mammoplasty, mastopexy, mastopexy with implants, augmentation mammoplasty in previously obese women after gastroplasty, and secondary mammoplasty. It is based on prior knowledge of the ligamentous suspension of the breast and makes it possible to obtain good vascularization, safety, and function in NAC flaps.
All records obtained are very recent (<1 year postsurgery). The group of patients operated is very heterogeneous (resection of 58-2350 g) and involves mainly cases of aesthetic mammoplasty (resection of breast tissue <700 g) for mild and moderate ptosis.
In this work, we chose to quantify the amount of skin resected in the vertical/cranial-caudal dimension by calculating the difference between the pre- and postoperative measurements, as an attempt to increase the comparability/reproducibility of data for future case series. The classification of mammary ptosis into grades I, II, and III, on the basis of the inframammary fold, does not precisely estimate the extent of mobilization of the NAC, as well as the exact size of the lower pole of the breast. This makes it very difficult to compare results between the different mammoplasty techniques and the diagnosis of pseudoptosis (nipple-inframammary fold distance >8 cm).
The sensitivity of the NAC is known to present a statistically insignificant change after mammoplasty. A number of cases with >2 years follow-up after mammoplasty showed normal NAC sensitivity18-20. The variation in methods affects the comparability of results in the literature. However, in this small and recent series of cases, it is worth noting that most patients (80%) reported normal sensitivity of the breasts, without any subjective change in the immediate postoperative period. Great vitality of the NAC flap, with no necrosis or ischemia, was also observed in 97.5% of cases. Indeed, in two patients, milk secretion from the NAC was observed after the suture of breast flap. Partial suffering of one areola was observed, originally 50 cm from the suprasternal notch, with good vitality observed until the moment before the suture. One of the explanations could be a possible compression or compartment syndrome of the NAC after the suture (see Figure 4).
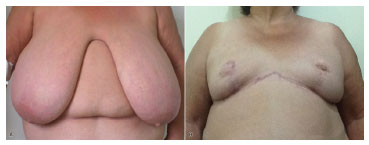
Figure 4. Preoperative and postoperative images.
The fascioadenocutaneous areolar flap is independent of dermal pedicles and presents a large detachment, which ensures a wide range of motion of the NAC. In this technical variation, to ensure maximum vascularization, the superior dermal pedicle is preserved. This can be considered an oblique bipedicled or multipedicled flap, based on the two main neurovascular systems of the NAC.
The wedge resection in the lateral and upper portion of the breast, unlike the central wedge, from the classic studies of the NAC dermal flap (Pitanguy), determines the elimination of the lateral excess of breasts, a common complaint in all patients. Regardless of the size of the breast tissue resected, no commitment to the vitality of the NAC flap occurs, which ensures versatility and safety (see Figure 5).
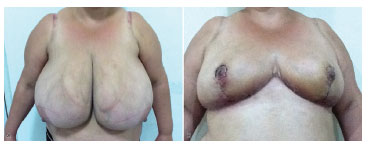
Figure 5. Preoperative and postoperative images.
In addition to the clinical application of the concept of the ligamentous suspension of the breast, at the time of constructing the new breast cone, the fascioadenocutaneous areolar flap can also simulate the effect of a breast implant or a breast fill flap12, which has the advantage of maintaining an intact areola-glandular unit.
CONCLUSION
In this series of cases, the fascioadenocutaneous areolar flap showed viability with preservation of function, safety, and versatility. The operated patients were highly satisfied with the results with regard to the shape, volume, and symmetry of the breasts. To date, this method has not presented any major complications, such as loss of the NAC. Up to now, this flap is applied in cases of mastopexy; secondary mammoplasty; and for slight, moderate, and large breast hypertrophy. This flap may be an additional technical option in mammoplasty.
REFERENCES
1. Ferreira MC. Evaluation of results in aesthetic plastic surgery: preliminary observations on mammaplasty. Plast Reconstr Surg. 2000;106(7):1630-5, discussion 1636-9. http://dx.doi.org/10.1097/00006534-200012000-00032. PMid:11129197
2. Hudson DA. Some thoughts on choosing a technique in breast reduction. Plast Reconstr Surg. 1998;102(2):554-7. http://dx.doi.org/10.1097/00006534-199808000-00043. PMid:9703098
3. Graf R, Biggs TM. In search of better shape in mastopexy and reduction mammoplasty. Plast Reconstr Surg. 2002;110(1):309-17, discussion 318-22. http://dx.doi.org/10.1097/00006534-200207000-00053. PMid:12087273
4 Würinger E, Mader N, Posch E, Holle J. Nerve and vessel supplying ligamentous suspension of the mammary gland. Plast Reconstr Surg. 1998;101(6):1486-93. http://dx.doi.org/10.1097/00006534-199805000-00009. PMid:9583477
5. Hidalgo DA, Elliot LF, Palumbo S, Casas L, Hammond D. Current trends in breast reduction. Plast Reconstr Surg. 1999;104(3):806-15, quiz 816, discussion 817-8. http://dx.doi.org/10.1097/00006534-199909010-00031. PMid:10456536
6. Rohrich RJ, Thornton JF, Sorokin ES. Recurrent mammary hyperplasia: current concepts. Plast Reconstr Surg. 2003;111(1):387-93, quiz 394. http://dx.doi.org/10.1097/00006534-200301000-00070. PMid:12496611
7. Hudson DA, Skoll PJ. Repeat reduction mammaplasty. Plast Reconstr Surg. 1999;104(2):401-8. http://dx.doi.org/10.1097/00006534-199908000-00013. PMid:10654683
8. Spear SL, Howard MA. Evolution of the vertical reduction mammaplasty. Plast Reconstr Surg. 2003;112(3):855-68, quiz 869. http://dx.doi.org/10.1097/01.PRS.0000072251.85687.1B. PMid:12960869
9. Berthe JV, Massaut J, Greuse M, Coessens B, De Mey A. The vertical mammaplasty: a reappraisal of the technique and its complications. Plast Reconstr Surg. 2003;111(7):2192-9, discussion 2200-2. http://dx.doi.org/10.1097/01.PRS.0000062621.83706.88. PMid:12794459
10. Chiari A Jr. The L short-scar mammaplasty: a new approach. Plast Reconstr Surg. 1992;90(2):233-46. http://dx.doi.org/10.1097/00006534-199290020-00011. PMid:1631215
11. Chiari A Jr. The L short-scar mammaplasty: 12 years later. Plast Reconstr Surg. 2001;108(2):489-95. http://dx.doi.org/10.1097/00006534-200108000-00032. PMid:11496194
12. Ribeiro L, Accorsi A Jr, Buss A, Marcal-Pessoa M. Creation and evolution of 30 years of the inferior pedicle in reduction mammaplasties. Plast Reconstr Surg. 2002;110(3):960-70. http://dx.doi.org/10.1097/00006534-200209010-00038. PMid:12172167
13. Losee JE, Caldwell EH, Serletti JM. Secondary reduction mammaplasty: is using a different pedicle safe? Plast Reconstr Surg. 2000;106(5):1004-8, discussion 1009-10. http://dx.doi.org/10.1097/00006534-200010000-00007. PMid:11039371
14. Würinger E. Refinement of the central pedicle breast reduction by application of the ligamentous suspension. Plast Reconstr Surg. 1999;103(5):1400-10. http://dx.doi.org/10.1097/00006534-199904020-00008. PMid:10190436
15. Würinger E. Secondary reduction mammaplasty. Plast Reconstr Surg. 2002;109(2):812-4. http://dx.doi.org/10.1097/00006534-200202000-00064. PMid:11818875
16. Datta G, Carlucci S. Selective breast reduction: a personal approach with a central-superior pedicle. Plast Reconstr Surg. 2009;123(2):433-42. http://dx.doi.org/10.1097/PRS.0b013e3181954c9a. PMid:19182599
17. Hamdi M, Van Landuyt K, Tonnard P, Verpaele A, Monstrey S. Septum-based mammaplasty: a surgical technique based on Würinger's septum for breast reduction. Plast Reconstr Surg. 2009;123(2):443-54. http://dx.doi.org/10.1097/PRS.0b013e318196b852. PMid:19182600
18. Mofid MM, Dellon AL, Elias JJ, Nahabedian MY. Quantitation of breast sensibility following reduction mammaplasty: a comparison of inferior and medial pedicle techniques. Plast Reconstr Surg. 2002;109(7):2283-8. http://dx.doi.org/10.1097/00006534-200206000-00018. PMid:12045551
19. Gonzalez F, Brown FE, Gold ME, Walton RL, Shafer B. Preoperative and postoperative nipple-areola sensibility in patients undergoing reduction mammaplasty. Plast Reconstr Surg. 1993;92(5):809-14, discussion 815-8. http://dx.doi.org/10.1097/00006534-199392050-00005. PMid:8415962
20. Harbo SO, Jørum E, Roald HE. Reduction mammaplasty: a prospective study of symptom relief and alterations of skin sensibility. Plast Reconstr Surg. 2003;111(1):103-10, discussion 111-2. http://dx.doi.org/10.1097/00006534-200301000-00017. PMid:12496570
1. Assistant Physician, Clínica de Cirurgia Plástica, Hospital Felício Rocho, Belo Horizonte, MG, Brazil
2. Director of the Plastic Surgery Service, Hospital Felício Rocho, Belo Horizonte, MG, Brazil
Institution: Work carried out at the Hospital Felício Rocho, Belo Horizonte, MG, Brazil.
Corresponding author:
Gustavo Moreira Costa de Souza
Plastic Surgery Service, Hospital Felício Rocho
Rua dos Timbiras, 3642 (conj. 504)
Belo Horizonte, MG, Brazil CEP 30140-062
E-mail: gustavomcsouza@gmail.com
Article received: August 15, 2011.
Article accepted: August 3, 2014.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license












