ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Sentinel lymph node evaluation in the treatment of cutaneous melanoma: systematization of a technique using lymphoscintigraphy and patent blue
Avaliação do linfonodo sentinela no tratamento do melanoma cutâneo: sistematização de técnica com linfocintigrafia e azul patente
Original Article -
Year2013 -
Volume28 -
Issue
3
Marcelo Marafon Maino
ABSTRACT
BACKGROUND: Melanoma incidence has been increasing worldwide. For localized melanoma, the status of the sentinel lymph node is the most important prognostic factor. This study aimed to evaluate the systematization of a technique to identify the sentinel lymph node anatomically by using lymphoscintigraphy and patent blue.
METHOD: A total of 12 cases were studied between March 2009 and March 2012. The treatment protocol followed criteria established by the Brazilian Group of Melanoma. Patients were evaluated for age, sex, thickness of the primary lesion, localization of drainage site of the sentinel lymph node, and presence of metastases.
RESULTS: The majority of patients were male and the mean age was 49.7 years. The types of thickness most frequently found were Breslow thickness 0-1 mm and Clark level II. All of the dissected lymph nodes were stained with patent blue. Histological and immunohistochemical analysis of the lymph nodes were negative for metastases.
CONCLUSIONS: The combined use of lymphoscintigraphy and patent blue allows for the precise localization of the sentinel lymph node, with a fast learning curve for surgeons, and low operative morbidity.
Keywords:
Sentinel lymph node. Melanoma. Lymphoscintigraphy. Patent blue.
RESUMO
INTRODUÇÃO: A incidência do melanoma vem aumentando em todo o mundo. O status do linfonodo sentinela é o fator prognóstico mais importante para o melanoma localizado. Este estudo teve como objetivo avaliar a sistematização da técnica de identificação anatômica do linfonodo sentinela com o uso de linfocintigrafia e azul patente.
MÉTODO: Foram estudados 12 casos no período de março de 2009 a março de 2012. O protocolo de tratamento seguiu os critérios do Grupo Brasileiro de Melanoma. Os pacientes foram avaliados quanto a idade, sexo, espessura da lesão primária, localização dos sítios de drenagem do linfonodo sentinela, e presença de metástases.
RESULTADOS: A maioria dos pacientes era do sexo masculino e a média de idade era de 49,7 anos. Em relação à espessura, os tipos mais encontrados foram 0-1 mm de Breslow e nível II de Clark. Todos os linfonodos dissecados foram corados por azul patente. A análise histológica e imuno-histoquímica dos linfonodos foi negativa para metástases.
CONCLUSÕES: O uso combinado de linfocintigrafia e azul patente permite a localização precisa do linfonodo sentinela, com rápida curva de aprendizado e baixa morbidade operatória.
Palavras-chave:
Biópsia de linfonodo sentinela. Melanoma. Linfocintigrafia. Azul patente.
INTRODUCTION
Despite representing only 3% to 4% of malignant cutaneous tumors, melanoma is the most important skin cancer. This is due to high mortality rates and a considerable increase over the last decades in melanoma incidence worldwide. In 2012, the estimated number of new cases of melanoma for Brazil was 6,230, with the highest rates estimated for men and women in Rio Grande do Sul (6.71 per 100 thousand men and 6.36 per 100 thousand women)1. Melanoma is curable and prognosis is good when detected at an early stage.
Treatment for primary melanoma of the skin is initiated by resection of the tumor with a wide margin. In cases of localized disease, the resection may be curative; however, the possibility of ganglion involvement increases for lesions of greater thickness, reaching 20% for patients with melanomas of intermediate Breslow thickness (1-4 mm)2. Removal of the lymphatic chain may be performed to complement treatment in these patients; however, its indication is questioned because aside from being a morbidity factor there is no impact on survival.
Sentinel lymph node (SLN) biopsy, initially described by Morton et al.3, has been widely employed in melanoma grading and prognosis. The SLN corresponds to the first lymph node of the lymphatic basin that receives drainage from a specific region. This almost always corresponds to the first site of tumor implantation as dissemination occurs in an ordered and sequential manner. In 1999, Gershenwald et al.4 conducted a multi-institutional study of Stage I and II patients, concluding that the presence of metastases in lymph nodes is the most important prognostic factor in patients with melanoma. This study aimed to evaluate the systematization of using lymphoscintigraphy and patent blue for SLN anatomic identification.
METHOD
A retrospective study of 12 patients who had undergone surgery between March 2009 and March 2012 was conducted. All cases were diagnosed by dermatologists through excisional biopsy. Guidelines established by the Brazilian Group of Melanoma (GBM) were used as inclusion criteria for SLN biopsy. These included the following: absence of regional or distant metastasis; primary lesions with Breslow thickness > 0.76 mm and < 0.76 mm, as long as associated with Clark levels IV or V; and/or ulceration and/or significant signs of regression5. Patients were evaluated for the following factors: age, sex, thickness of the primary lesion, location of drainage site of the SLN, and presence of metastases.
The same sequence was performed in all of the patients:
Lymphoscintigraphy performed by the same Radiology Service, from 4 to 12 hours prior to surgery, with intradermal injection of 1 mCi of 99mTc-dextran in 1 ml of saline solution around the biopsy scar (Figure 1).

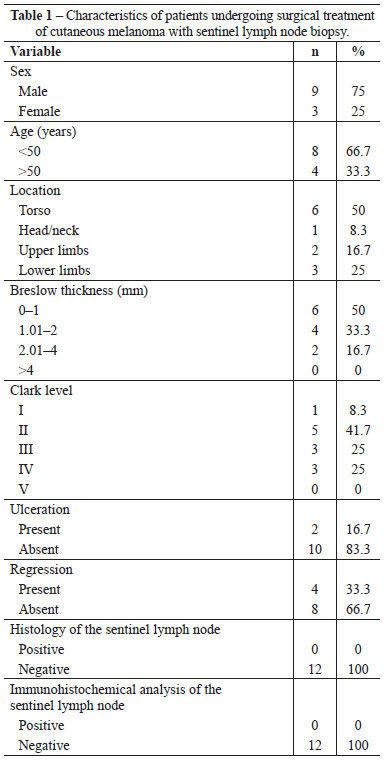
Figure 1 - Pre-operative lymphoscintigraphy showing single drainage for the right axillary region (primary lesion in the back).
Marking the increase of the margin area, having as a parameter the scar of the primary lesion excision (Figure 2).
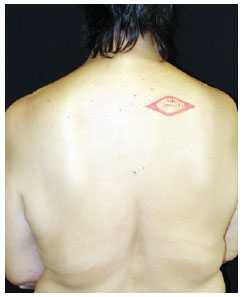
Figure 2 - Marking the area of margin increase, with the scar of the primary lesion excision serving as the parameter.
Marking the site(s) for surgical exploration of the SLN (Figure 3).
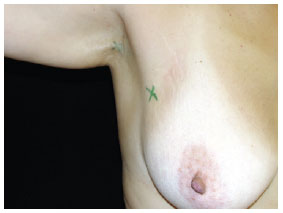
Figure 3 - Marking the site of the sentinel lymph node (right axillary region) for surgical exploration.
Induction of general anesthesia.Intradermal, pericicatricial application of 1 ml patent blue dye up to the demarcation limit of the margin increase (Figure 4).
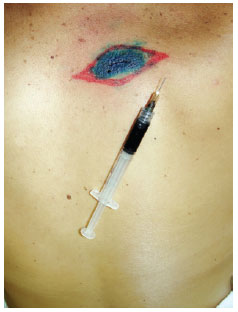
Figure 4 - Intradermal, pericicatricial injection of 1 ml of patent blue up to the demarcation of the margin increase.
Search for SLN(s) stained with patent blue using a transoperative manual gamma radiation detector (Figure 5).
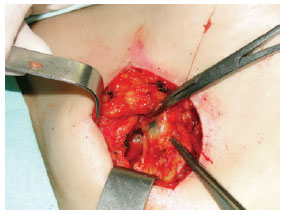
Figure 5 - Localization and dissection of the sentinel lymph node stained with patent blue (right axillary region).
Tissue ligation with absorbable sutures and excision of the stained lymph node(s).The search was finalized only after finding and counting, in the surgical bed, < 10% of the initial lymph node(s) ex-vivo.Subcutaneous and subdermal plane suture using an absorbable thread and skin with nylon 4.0 or 5.0.Margin increase of the primary lesion, reaching deep into the fascial plane.Outpatient hospital discharge.Request for anatomopathological and immunohistochemical exam for S-100 and HMB-45 proteins.Post-operative revisions at 7 days, 14 days, 30 days and 90 days (Figure 6).
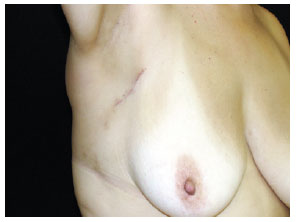
Figure 6 - Post-operative revision at 90 days.
RESULTS
A total of 12 patients were studied, with a mean age of 49.7 years (range: 28 to 74 years). The majority of the patients were male (75%). The most frequent location of the primary cutaneous lesion was the torso (50%). The most common types of thickness found were Breslow thickness 0-1 mm (50%) and Clark level II (41.7%; Table 1).
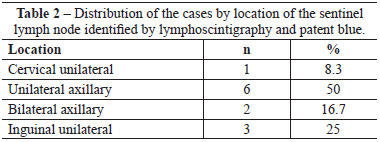
All of the SLNs stained with patent blue were identified in the patients. Two cases of double axillary drainage occurred (Figure 7) in patients whose primary lesion was localized to the torso (Table 2).
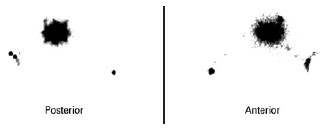
Figure 7 - Pre-operative lymphoscintigraphy showing double draining for the right and left axillary regions (primary lesion in the back).
In histological and immunohistochemical analysis, no lymph nodes showed metastases; thus, regional lymphadenectomy was not recommended.
With respect to operative morbidity, only 1 patient presented with a partial suture dehiscence in the margin increase of the primary site (lower limb). There was no need for a complementary procedure. In the sites of SLN biopsy, there were no complications (e.g. dehiscence, seroma, hematoma, infection). None of the patients showed an allergic reaction to the Tc-dextran or patent blue.
DISCUSSION
The SLN biopsy is indicated for patients diagnosed with melanoma without clinically evident lymph nodes, and which have a significant risk for micrometastasis. Regional lymphadenectomy is indicated without a SLN biopsy in cases where macroscopic clinical evidence of compromised lymph nodes and/or a positive result for melanoma based on aspiration biopsy exists.
The decision to perform a SLN biopsy should be carried out before local excision to increase margins begins, since lymphatic drainage may be altered in the increased resections or repairs using flap rotation 6.
This technique involves 4 procedures: pre-operative lymphoscintigraphy, trans-operative lymphatic mapping with patent blue, use of a transoperative manual detector of gamma radiation, and histopathologic diagnosis.
In 1993, Alex et al.7 proposed intradermal injection of colloidal sulfur labeled with Technetium-99m around the melanoma, followed by scintigraphy to identify the lymphatic drainage path of the tumor and SLN localization. The correct identification of the lymphatic chain by the pre-operative lymphoscintigraphy study is a strength of the method, mainly when the tumor is localized in ambiguous drainage areas8. In the present study, we found double lymphatic drainage (bilateralism) in 2 cases) indicating that we should not only consider the Sappey lines during clinical analysis9.
In 1996, Glass et al.10 reported the use of intra-operative lymphoscintigraphy associated with blue colorant to localize the SLN biopsy site. A radiation portable detector (gamma-probe) is used in the trans-operative period to direct the dissection of the SLN and, subsequently, confirm its removal. A pre-operative injection of patent blue in the lesion facilitates the identification of the lymphatic trajectory and the stained lymph node during surgery. Combining techniques minimizes great dissections, reduces post-operative complications and, in our case-by-case basis study, shows a fundamental 100% success rate in the localization of the SLN.
Besides the anatomopathological examination, it is recommended that an immunohistochemical examination with antibodies directed to the S-100 and HMB-45 antigens be performed in order to increase sensitivity in the detection of metastasis 11-14.
In 2010, Silva et al.15 performed a systematic review of observational studies to evaluate indications for SLN biopsy in thin melanomas. They concluded that the following results were statistically significant for confirming the presence of micrometastases: Breslow thickness > 0.75 mm, Clark level IV-V, mitotic index > 5/mm2 and absence of regression. Such findings corroborate the recommendation criteria used in this study.
CONCLUSIONS
The plastic surgeon should have adequate knowledge of the diagnosis, treatment, and monitoring of patients with cutaneous melanoma. In these stages, knowing when and how to perform the SLN biopsy in order to identify regional micrometastases is of fundamental importance for disease prognosis. The systematization of the combined use of lymphoscintigraphy and patent blue allows localization of the SLN with precision, with a fast learning curve for surgeons, and a low operative morbidity.
REFERENCES
1. Brasil. Ministério da Saúde. Estimativa 2012: incidência de câncer no Brasil. Instituto Nacional do Câncer. Rio de Janeiro: INCA; 2012.
2. Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner H, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;335(13):1307-17.
3. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392-9.
4. Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17(3):976-83.
5. Neves RI, Belfort FA, Brandão M, Silva DCP. Relatório final do consenso nacional sobre linfonodo sentinela (LNS) do Grupo Brasileiro de Melanoma. Acta Oncol Bras. 2003;23:499-503.
6. Wainstein AJA, Belfort FA. Conduta para o melanoma cutâneo. Rev Col Bras Cir. 2004;31(3):204-14.
7. Alex JC, Weaver DL, Fairbank JT, Rankin BS, Krag DN. Gamma-probe-guided lymph node localization in malignant melanoma. Surg Oncol. 1993;2(5):303-8.
8. Castro LGM, Duprat JP, Landman G. Dupla drenagem para cadeias linfonodais distintas, detectada por técnica de biópsia de linfonodo sentinela em pacientes com melanoma cutâneo: relato de dois casos. An Bras Dermatol. 2005;80(5):499-502.
9. Uren RF, Howman-Giles RB, Chung D, Thompson JF. Role of lymphoscintigraphy for selective sentinel lymphadenectomy. Cancer Treat Res. 2005;127:15-38.
10. Glass LF, Messina JL, Cruse W, Wells K, Rapaport D, Miliotes G, et al. The use of intraoperative radiolymphoscintigraphy for sentinel node biopsy in patients with malignant melanoma. Dermatol Surg. 1996;22(8):715-20.
11. Cochran AJ, Wen DR, Morton DL. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol. 1988;12(8):612-8.
12. Baisden BL, Askin FB, Lange JR, Westra WH. HMB-45 immunohistochemical staining of sentinel lymph nodes: a specific method for enhancing detection of micrometastases in patients with melanoma. Am J Surg Pathol. 2000;24(8):1140-6.
13. Duprat JP, Silva DC, Coimbra FJ, Lima IA, Lima EN, Almeida OM, et al. Sentinel lymph node biopsy in cutaneous melanoma: analysis of 240 consecutive cases. Plast Reconstr Surg. 2005;115(7):1944-51.
14. de la Fuente-García A, Ocampo-Candiani J. Melanoma cutáneo. Gac Med Mex. 2010;146(2):126-35.
15. Silva FB, Oliveira Filho RS, Iared W, Atallah AN, Santos IDAO, Ferreira LM. Indicações de biópsia do linfonodo sentinela em melanomas finos. Einstein. 2010;8(2):235-40.
Master in Surgery, full member of the Brazilian Society of Plastic Surgery (SBCP), Porto Alegre, RS, Brazil
Correspondence to:
Marcelo Marafon Maino
Avenida Soledade, 569 - cj. 1.106 - Petrópolis
Porto Alegre, RS, Brazil - CEP 90470-340
E-mail: mmaino@terra.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: July 26, 2012
Article accepted: December, 15, 2013
Work performed in the Mãe de Deus Health System, Porto Alegre, RS, Brazil.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license