ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Cost comparison between enoxaparin and new oral anticoagulants dabigatran and rivaroxaban in venous thromboembolism prophylaxis
Comparação de custo da enoxaparina e dos novos anticoagulantes orais dabigatran e rivaroxaban na profilaxia do tromboembolismo venoso
Original Article -
Year2013 -
Volume28 -
Issue
3
André Toshiaki Toda Nishimura1; André Domingues Pereira2; Antônio Egídio Rinaldi3; Sabrina Garnier Khalil1; Diogo Ferreira Pinto Krohling1
ABSTRACT
BACKGROUND: Venous thromboembolism, an important complication that is easily prevented during surgery, is among the few clinical entities wherein the use of prophylaxis has been found to be effective, such as in the case of antibiotic prophylaxis for the prevention of surgical site infections. The objectives of prophylaxis are to reduce the incidence of deep vein thrombosis, minimize the risk of long-term complications such as chronic venous insufficiency and pulmonary hypertension, and prevent death caused by pulmonary embolism. In this study, we aimed to provide current information to plastic surgeons on the therapeutic options for prophylaxis of venous thromboembolism as well as to compare treatment costs of the drugs enoxaparin, dabigatran, and rivaroxaban.
METHOD: For comparison, the prophylaxis regimen was started 6 hours after the surgery and continued for 10 days. The end user price was obtained from an internet search that included leading pharmacy chains.
RESULTS: The patient costs of the new oral anticoagulants dabigatran and rivaroxaban were lower than that of enoxaparin.
CONCLUSIONS:The cost of venous thromboembolism prophylaxis consisting of the new oral anticoagulants dabigatran and rivaroxaban is lower than that of low molecular weight heparin.
Keywords:
Thromboembolism. Venous thromboembolism/prevention & control. Anticoagulants.
RESUMO
INTRODUÇÃO: O tromboembolismo venoso é uma complicação importante altamente evitável em cirurgia, sendo uma das poucas entidades clínicas em medicina passíveis de profilaxia comprovadamente eficiente, assim como ocorre na profilaxia antibiótica das infecções cirúrgicas. A profilaxia tem por objetivo diminuir a incidência de trombose venosa profunda, minimizar os riscos das complicações a longo prazo da insuficiência venosa crônica e hipertensão pulmonar, bem como prevenir a morte consequente de embolia pulmonar. Este estudo tem por objetivo atualizar os cirurgiões plásticos quanto às medicações que podem ser adotadas na profilaxia do tromboembolismo venoso, bem como comparar o custo dessas medicações (enoxaparina, dabigatran e rivaroxaban).
MÉTODO: Para efeito de comparação foi adotado um esquema de profilaxia iniciado 6 horas após o fim da cirurgia e mantido por 10 dias. A pesquisa foi realizada na internet entre as principais redes farmacêuticas, levando em conta o custo para o consumidor.
RESULTADOS: Os novos anticoagulantes orais dabigatran e rivaroxaban apresentaram custo menor para o paciente.
CONCLUSÕES: Os novos anticoagulantes orais dabigatran e rivaroxaban possuem custo menor na profilaxia do tromboembolismo venoso em comparação à heparina de baixo peso molecular.
Palavras-chave:
Tromboembolia. Tromboembolia venosa/prevenção & controle. Anticoagulantes.
INTRODUCTION
Venous thromboembolism (VTE), a disease that includes both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a serious surgical complication that is relevant for all plastic surgeons1-13. Over the past 25 years, several studies have repeatedly proven the efficacy of antithrombotic prophylaxis14,15.
The objectives of prophylaxis include reducing the incidence of DVT, minimizing the risk of long-term complications such as chronic venous insufficiency and pulmonary hypertension, and preventing death caused by PE2.
Without prophylaxis, one in four patients aged >40 years of age will develop VTE when they undergo major surgery that lasts for >1 hour2. There are 250,000 cases of VTE annually in the United States16. PE is responsible for 150,000 deaths per year and for 5% of all perioperative deaths16.
In plastic surgery, the highest rates of VTE (1.2%) and PE (0.8%) are associated with abdominoplasty; when this procedure is combined with intra-abdominal procedures such as hysterectomy, the incidence of PE increases to 6.6%16. The rates of VTE and PE after liposuction are 0.6% and 1.1%, respectively5,16, while those after rhytidoplasty are 0.35% for VTE and 0.14% for PE4,5,16.
Antithrombotic prophylaxis can be achieved mechanically or pharmacologically2. The mechanical methods include elastic compression stockings and intermittent pneumatic compression and are reserved for low-risk patients or those at high risk of bleeding17. Pharmacological methods are generally selected for moderate- to high-risk patients or for surgical procedures that are more commonly associated with VTE17. The drugs used in antithrombotic prophylaxis include unfractionated heparin, low molecular weight heparin, fondaparinux, and new oral anticoagulants17.
Dabigatran was discovered among a panel of chemicals with similar structures to that of the benzamidine-based thrombin inhibitor N-α-(2-naphthylsulfonylglycyl)-4-amidinophenylalanine piperidide, which has been known to be as a powerful inhibitor of various serine proteases, specifically thrombin but also trypsin, since the 1980s18. The addition of an ethyl ester and hexyloxycarbonyl carbamide hydrophobic side chains led to the development of the orally absorbed pro-drug dabigatran etexilate (BIBR 1048). Dabigatran, an oral anticoagulant drug that belongs to the direct thrombin inhibitors class, is manufactured by the pharmaceutical company Boehringer Ingelheim and has been approved for sale by the European Medicines Agency since 2008, US Food and Drug Administration since 2010, and ANVISA since 201118.
Rivaroxaban (BAY 59-7939), an oral anticoagulant developed by Bayer Schering Pharma, is oxazolidinone optimized for inhibiting both free Factor Xa and Factor Xa bound in the prothrombinase complex18. As a selective direct Factor Xa inhibitor, rivaroxaban interrupts the intrinsic and extrinsic blood coagulation cascade pathways, thus inhibiting thrombin formation and thrombi development18. Rivaroxaban was the first direct Factor Xa inhibitor to be introduced in the market18. Both drugs are still in the Phase IV (post-marketing surveillance) stage of development, a period in which any detected adverse effect or toxicity should be reported to the authorities18.
The development of new oral anticoagulants, which are more effective and safer than the recommended dose of enoxaparin for VTE prophylaxis19, has revolutionized the prophylaxis and treatment of VTE.
For VTE prophylaxis, dabigatran is given at oral doses of 150 mg daily. The drug is available in the market under the trade name Pradaxa in three formats: packs containing ten or thirty 75-mg tablets; packs containing ten or sixty 110-mg tablets; and packs containing ten, thirty, or sixty 150-mg tablets.
Rivaroxaban is given at oral doses of 10 mg daily. The drug is available in the market under the trade name Xarelto in two formats: packs containing ten or thirty 10-mg tablets; and packs containing fourteen or twenty-eight 15- or 20-mg tablets.
Enoxaparin is given subcutaneously at a dose of 40 mg daily that requires adjustment in obese patients (1 mg/kg/day). The drug is available on the market under the trade names Clexane, Cutenox, Enoxalow, Enoxil, Heptron, and Versa in packs containing two or ten 40-, 60-, 80-, or 100-mg syringes.
The objective of this study is to provide current information to plastic surgeons on the therapeutic options for use in VTE prophylaxis as well as to compare treatment costs of the drugs enoxaparin, dabigatran, and rivaroxaban.
METHODS
For cost comparison purposes, the prophylaxis regimen was started 6 hours after surgery and was continued for 10 days. An internet search was performed and included a total of 12 large pharmacy chains. The two leading companies had pharmacies in eight or nine states The other chains had a home delivery system in the following states: Bahia, Distrito Federal, Espírito Santo, Goiás, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Paraná, Rio de Janeiro, Rio Grande do Sul, Santa Catarina, and São Paulo; in this case, the final price for the end user was considered.
The mean price was obtained by adding all values obtained from the search and divided by the number of results found (dabigatran, 12 results; rivaroxaban, eight results; and enoxaparine, 12 results). The mean unit price was obtained by dividing the mean price by the number of syringes or tablets used during the 10-day treatment period.
RESULTS
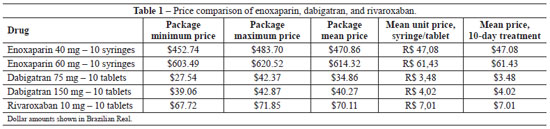
Cost savings of 88.65% and 85.19% were observed when the cost of treatment using 75 mg dabigatran was compared to that of 60 mg or 40 mg enoxaparin, respectively. In the case of treatment using 150 mg dabigatran, cost savings corresponded to 93.44% of that with 60 mg enoxaparin and to 91.44% of that with 40 mg enoxaparin.
Cost savings of 88.58% and 85.11% were observed when the cost of treatment using 10 mg rivaroxaban was compared to that of 60 mg or 40 mg enoxaparin, respectively. Thus, the costs of the new oral anticoagulants (dabigatran and rivaroxaban) are lower than that of enoxaparin (Table 1).
DISCUSSION
Although there is consensus among plastic surgeons supporting the use of mechanical methods (early deambulation, elastic compression stockings, and intermittent pneumatic compression) in antithrombotic prophylaxis, the use of pharmacological prophylaxis is almost considered taboo.
Some plastic surgeons question the methodology, conflicts of interest of the authors who publish on this subject, and the increased risk of hematoma. However, a large number of randomized double-blinded studies performed over the past 25 years with a wide range of surgical procedures has clearly demonstrated the benefits of chemoprophylaxis in the prevention of VTE.
Although different protocols suggest the use of pharmacological prophylaxis, there is no consensus for a single specific protocol. It has been observed, however, that all protocols take into consideration the patient's surgical stress response (low, moderate, or high risk) and risk stratification for (low, moderate, or high).
According to the leading specialists in the area of antithrombotic prophylaxis, the main issue associated with these protocols is the fact that one cannot add the rates, which is the case for the scoring system reported in most of the protocols. The rates should be calculated separately for each type of procedure; subsequently, a prophylaxis regimen is implemented as indicated. Approximately 6% of the patients who undergo total hip arthroplasty, a procedure that typically requires pharmacological prophylaxis, are at risk of developing PE20,21. In plastic surgery, the same rate is obtained when dermolipectomy combined with hysterectomy is performed. This is an example of a procedure from our specialty that typically requires pharmacological prophylaxis since it carries a risk of PE equivalent to 6.6%16.
Some protocols suggest that prophylaxis should be initiated 6 hours before the surgical procedure. However, prophylaxis is contraindicated for patients who are subjected to epidural anesthesia. For cost comparison purposes, the protocol used in this study assumed that prophylaxis started 6 hours after surgery, as it has been shown that post-surgical bleeding does not occur when enoxaparin in initiated during this period22; additionally, there would be no issues associated with the selected form of anesthesia.
Plastic surgeons are reluctant to use chemoprophylaxis in rhytidoplasties because of the risk of hematoma since bleeding can be catastrophic and lead to profound changes in the aesthetic result. Studies have demonstrated that the risks of DVT and PE are 0.35% and 0.14%, respectively4,5,16, despite a mean surgical time of 6 hours. This procedure is associated with a low risk for DVT and PE, and is an exception since it relates to the use of pharmacological prophylaxis (mechanical methods are always used) unless the patient is at high risk for VTE.
Given that specific antidotes to reverse the anticoagulant activity of new oral anticoagulants are not available, we propose the initiation of the protocol 6 hours after surgery because there is no need for surgical re-intervention. In cases of light bleeding, the best alternative is to discontinue anticoagulant treatment due to the short half-life that is usually associated with these drugs (range, 7-14 hours). No effective measures are available in situations in which there is severe bleeding, but treatment options include the use of recombinant activated factor VII and activated prothrombin complex concentrates.
For cost comparison purposes, the suggested prophylaxis regimen was continued for only 10 days. Although there are case reports in the literature in which the prophylaxis use exceeded 10 days, there is no consensus of a specific duration of the postoperative prophylaxis. However, it is well known that mortality rates due to PE are higher 3-7 days after surgery and that 10% of cases of symptomatic PE are fatal within the first hour from the onset of symptoms23. Moreover, the drugs are sold in packs containing 10 units.
It is important to mention that hospitals have adopted the questionable scoring system to predict operative risk and that low-risk surgeries are often classified as moderate- or even high-risk procedures because of the surgical duration. In case VTE complications occur, it is difficult to argue against the questionable protocols; thus, there should be a joint effort among plastic surgeons to prevent hospitals from adopting these recommendations in favor of medical autonomy.
Despite the advantages of the new oral anticoagulants in VTE prophylaxis, which include convenient oral administration, lower cost, higher efficacy, and a similar safety profile compared to the recommended dose of enoxaparin, there are no effective antidotes to reverse the anticoagulant activity of these drugs19.
CONCLUSIONS
The study findings suggest that antithrombotic prophylaxis should be used. The costs of the new oral anticoagulants dabigatran and rivaroxaban are lower than that of enoxaparin; however, there are no effective antidotes to reverse the anticoagulant activity of these drugs.
However, additional studies should be performed to better understand the incidence of DVT and PE according to specific surgical procedures to provide more reliable chemoprophylaxis protocols for our specialty.
REFERENCES
1. Comerota AJ, Stewart GJ. Current concepts in etiology of postoperative deep vein thrombosis. In: Callow AD, Ernst CB, eds. Vascular surgery: theory and practice. Stanford: Appleton & Lange; 1999. p. 1453-61.
2. Sandri JL. Profilaxia do tromboembolismo em cirurgia plástica. In: Carreirão S, Cardim V, Goldenberg D, eds. Cirurgia Plástica. Sociedade Brasileira de Cirurgia Plástica. São Paulo: Atheneu; 2005. p. 119-26.
3. McDevitt NB. Deep vein thrombosis prophylaxis. American Society of Plastic and Reconstructive Surgeons. Plast Reconstr Surg. 1999;104(6):1923-8.
4. Reinisch JF, Bresnick SD, Walker JW, Rosso RF. Deep venous thrombosis and pulmonary embolus after face lift: a study of incidence and prophylaxis. Plast Reconstr Surg. 2001;107(6):1570-7.
5. Rohrich RJ, Rios JL. Venous thromboembolism in cosmetic plastic surgery: maximizing patient safety. Plast Reconstr Surg. 2003;112(3):871-2.
6. Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114(3):43E-51E.
7. Most D, Kozlow J, Heller J, Shermak MA. Thromboembolism in plastic surgery. Plast Reconstr Surg. 2005;115(2):20e-30e.
8. The Doctors Company. Deep venous thrombosis and pulmonary embolism in plastic surgery office procedures. Disponível em: http://www.thedoctors.com. Acesso em: 31/3/2012.
9. Conroy FJ, Thornton DJ, Mather DP, Srinivasan J, Hart NB. Thromboembolic prophylaxis in plastic surgery: a 12-year follow up in the UK. J Plast Reconstr Aesthet Surg. 2006;59(5):510-4.
10. Newall G, Ruiz-Razura A, Mentz HA, Patronella CK, Ibarra FR, Zarak A. A retrospective study on the use of a low-molecular-weight heparin for thromboembolism prophylaxis in large-volume liposuction and body contouring procedures. Aesthetic Plast Surg. 2006;30(1):86-97.
11. Young VL, Watson ME. The need for venous thromboembolism (VTE) prophylaxis in plastic surgery. Aesthet Surg J. 2006;26(2):157-75.
12. Green D. VTE prophylaxis in aesthetic surgery patients. Aesthet Surg J. 2006;26(3):317-24.
13. Broughton G 2nd, Rios JL, Rohrich RJ, Brown SA. Deep venous thrombosis prophylaxis practice and treatment strategies among plastic surgeons: survey results. Plast Reconstr Surg. 2007;119(1):157-74.
14. Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318(18):1162-73.
15. Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88(7):913-30.
16. Most D, Kozlow J, Heller J, Shermak MA. Thromboembolism in plastic surgery. Plast Reconstr Surg. 2005;115(2):20e-30e.
17. Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. American College of Chest Physicians. Prevention of VTE in non-orthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis. 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S-77S.
18. Wikipedia. Dabigatran/Rivaroxaban. Disponível em: http://www.en.wikipedia.org Acesso em: 31/3/2012.
19. Nieto JA, Espada NG, Merino RG, González TC. Dabigatran, rivaroxaban and apixaban versus enoxaparin for thomboprophylaxis after total knee or hip arthroplasty: pool-analysis of phase III randomized clinical trials. Thromb Res. 2012;130(2):183-91.
20. Cassone A, Viegas AC, Sguizzatto GT, Cabrita HABA, Aquino MA, Furlaneto ME, et al. Trombose venosa profunda em artroplastia total de quadril. Rev Bras Ortop. 2002;37(5):153-61.
21. Bowler DJ, Bale E, O'Byrne J. Factor V Leiden: prevalence and thromboembolic complications after total hip replacement in Ireland. Ir J Med Sci. 2007;176(4):273-7.
22. Bailey SH, Pannucci C, Dreszer G, Fischer CF, Jaber C, Jaber RM, et al. Post-operative enoxaparin does not increase risk for re-operative hematoma in plastic surgery patients: preliminary results of the Psefs Venous Thromboembolism Prevention Study (Vteps). Plast Reconstr Surg. 2011;127:12.
23. Seruya M, Baker SB. MOC-PS(SM) CME article: venous thromboembolism prophylaxis in plastic surgery patients. Plast Reconstr Surg. 2008;122(3 Suppl):1-9.
1. Plastic surgeon, associate member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery - SBCP), São Paulo, SP, Brazil
2. Physician with a university degree in Hematology and Hemotherapy obtained from the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (Hospital das Clínicas, School of Medicine, University of São Paulo), specialist in Hematology and Hemotherapy by the Sociedade Brazileira de Hematologia e Hemoterapia (Brazilian Society of Hematology and Hemotherapy), São Paulo, SP, Brazil
3. Plastic surgeon, full member of the SBCP, lecturer at Serviço de Cirurgia Plástica Professor Doutor Oswaldo de Castro (Plastic Surgery Service Professor Doutor Oswaldo de Castro), São Paulo, SP, Brazil
Correspondence to:
André Toshiaki Toda Nishimura
Alameda Lorena, 2.015 - Cerqueira César
São Paulo, SP, Brazil - CEP 01424-002
E-mail: andre_nishi@me.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: April 17, 2012
Article accepted: July 1, 2012
This study was performed at the Serviço de Cirurgia Plástica Professor Doutor Oswaldo de Castro (Plastic Surgery Service Professor Doutor Oswaldo de Castro), São Paulo, SP, Brazil.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license