ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Treatment of breast ptosis and hypomastia using the superomedial pedicle technique of mammaplasty combined with breast augmentation
Tratamento da ptose mamária e hipomastia utilizando técnica de mamoplastia com pedículo súpero-medial e implante mamário
Original Article -
Year2012 -
Volume27 -
Issue
4
Alexandre Wada1; Lincoln Saito Millan2; Samuel Terra Gallafrio2; Rolf Gemperli3; Marcus Castro Ferreira4
ABSTRACT
BACKGROUND: One-stage treatment of breast ptosis with hypomastia using mastopexy techniques combined with breast augmentation is often believed to have the potential for more serious complications than when the procedures are performed separately. However, available data show that the incidence of complications associated with the combined treatment is similar to that of both procedures performed separately. Several authors have developed standard and specific care techniques that facilitate the safe use of this combined technique. The aim of this study is to describe the surgical technique used for breast augmentation in patients with breast ptosis, as well as the incidence of complications and surgical revision.
METHODS: The incidence of complications and surgical revision was analyzed in 27 patients who underwent one-stage mastopexy combined with breast augmentation using the superomedial pedicle technique, between 2005 and 2010.
RESULTS: There were no immediate complications that required early reoperations. Three (11.1%) patients had slight dehiscence of the suture at the inverted-T junction, with spontaneous resolution. One (3.7%) patient developed capsular contracture 1 year after the operation. Four (14.8%) patients underwent scar revision procedures. A history of smoking was associated with a four-fold increase in the incidence of suture dehiscence and doubled the number of scar revision procedures; however, the difference was not statistically significant.
CONCLUSIONS: The mastopexy technique combined with breast augmentation using the superomedial pedicle technique was effective and safe for the treatment of breast ptosis with hypomastia.
Keywords:
Breast/surgery. Breast implantation. Mammaplasty.
RESUMO
INTRODUÇÃO: O tratamento da ptose mamária com hipomastia utilizando técnica de mastopexia associada a implante mamário em um tempo cirúrgico é frequentemente associado a potenciais complicações mais graves do que quando se utilizam os métodos separadamente. Na literatura, a incidência relatada de complicações é semelhante à de procedimentos realizados em dois tempos cirúrgicos. Vários autores descrevem padronizações e cuidados específicos que possibilitam a utilização dessa combinação de técnicas com segurança. O objetivo deste trabalho é relatar técnica operatória para aumento mamário em pacientes com ptoses mamárias, analisando a incidência de complicações e revisões cirúrgicas.
MÉTODO: Foi avaliada a incidência de complicações e revisões cirúrgicas de 27 pacientes submetidas a mastopexia com implante mamário em um tempo cirúrgico utilizando técnica de pedículo súpero-medial, operadas entre 2005 e 2010.
RESULTADOS: Não foram observadas complicações imediatas que levassem a reoperações precoces. Três (11,1%) pacientes apresentaram pequenas deiscências de sutura na junção do "T" invertido, com resolução espontânea. Uma (3,7%) paciente apresentou contratura capsular, 1 ano após a operação. Quatro (14,8%) pacientes foram submetidas a revisões cirúrgicas de cicatrizes. História prévia de tabagismo aumentou em 4 vezes a incidência de deiscências de suturas e, em 2 vezes, o índice de revisões cirúrgicas de cicatrizes, porém sem diferença estatisticamente significante.
CONCLUSÕES: A técnica de mastopexia associada a implante mamário utilizando técnica de pedículo súpero-medial foi eficaz e segura para o tratamento da ptose mamária com hipomastia.
Palavras-chave:
Mama/cirurgia. Implante mamário. Mamoplastia.
INTRODUCTION
The one-stage combination of mastopexy with breast augmentation was first described in the 1960s1,2. In the literature, this combination of mastopexy with breast augmentation is a controversial topic; some authors describe complications and unsatisfactory aesthetic results3-6, whereas other authors have described the standardization of techniques to increase the safety and efficacy of this combination procedure6-11.
Spear et al.5 described an increase in the incidence of serious complications with the one-stage mastopexy with breast augmentation, including complications such as infection, implant exposure, loss of sensitivity in the areola and nipple, nipple malposition, implant malposition, necrosis of the areola, necrosis of the skin, and poor quality of the scars. These authors state that, compared to mastopexy or breast augmentation alone, the one-stage combination of the 2 procedures increases the overall incidence of complications and can result in more serious complications.
One of the principal reasons for the complexity of this procedure is that, in this combination, practically all the variables that determine the aesthetic appearance of the breast are manipulated to some degree4,12.
Lindsey13 proposed the treatment of grade II ptotic breasts using breast implants alone, inserting them via the inferior periareolar access. According to the author, the periareolar access allows for a more appropriate placement of the implant, and thus, a better correction of the ptosis than that obtained by using the inframammary access.
In the reviewed literature, the reported rates of complications and surgical reviews after one-stage mastopexy with breast augmentation vary considerably, and they refer to different mastopexy techniques, different types of breast implants, and different follow-up periods, which makes interpretation of the results difficult4,14. Stevens et al.10 showed that a 14.6% surgical revision rate at 3.5 years in patients who had undergone mastopexy with breast augmentation was better as compared to the rates of surgical revisions in patients who had undergone mastopexy alone (8.6%) or breast augmentation alone (13%) over the same period. An increase in the probability of a revision was related to periareolar mastopexy that was performed with the insertion of saline-filled implants in patients who had a history of smoking. Nevertheless, according to the authors, "...this revision rate is far from the 100% reoperation rate required for a staged procedure." Seify et al.15 reporteda 6.8% rate of surgicalrevision over a period of 3.5 years after one-stage mastopexy with augmentation, and a 10.5% rate of complications. Gomes11 reported the use of the superior pedicle technique, with the implant positioned subglandularly; recurrent ptosis occurred in 7.4% of cases.
Karacaoglu12 reported the absence of surgical revisions over a period of 20.6 months after mastopexy with breast augmentation, using the vertical mastopexy technique, subpectoral positioning of the implant, and a sutured skin graft between the border of the pectoralis muscle and the thoracic wall, which acts as a sling or support for the implant. Spear et al.5 noted an 8.7% revision rate after mastopexy combined with breast augmentation, compared to a 1.7% revision rate after primary breast augmentation. The average time between mastopexy with augmentation and revision was 7 years. The rate of complications was 17%.
Cannon and Lindsey14 recommended the one-stage technique involving periareolar mastopexy combined with submuscular implant placement for the treatment of moderate ptosis. For a relocation of the areola by more than 4 cm, they suggest the use of either the vertical or inverted-T mastopexy technique alone, followed by breast augmentation in a second stage after 6 months. According to these authors, the risk of necrosis of the areolas, asymmetries, necrosis of the mammary tissue, and hypertrophic scars is thus reduced.
One-stage mastopexy combined with augmentation should be carefully assessed in patients who have severe secondary breast ptosis due to weight loss after bariatric surgery, because extensive mobilization of the areola is required, which results in long pedicles. In addition, while the patient was obese, the skin was subject to traction for a prolonged time, and its ability to support very large implants is reduced16-18.
Don Parsa et al.19 proposed a surgical approach for the correction of breast ptosis, using breast implants, which is based on the degree of ptosis. For mild ptosis (nipple above the inframammary fold, and mammary tissue less than 1 cm below the fold), these authors use subglandular or submuscular breast augmentation, depending on the amount of existing mammary tissue. For moderate ptosis (nipple above or at the level of the inframammary fold, and mammary tissue from 1 cm to 4 cm below the fold), they recommend breast augmentation alone, followed by some form of mastopexy 3 months later. For severe ptosis (nipple at the level or below the inframammary fold, and mammary tissue more than 4 cm below the fold), they recommend mastopexy in the first stage, followed by breast augmentation 3 months later.
Spear et al.5 recommend thorough preparation, planning, and care, particularly with regard to the position of the areola, the preservation of blood supply to the areola, and adequate skin closure tension.
Elliott20 described a one-stage circumareolar mastopexy technique with subpectoral breast augmentation for cases in which the distance from the suprasternal notch to the nipple is less than 24 cm.
Although there are opinions in favor of staged mastopexy and breast augmentation, mastopexy performed on patients who have previously undergone a breast augmentation with implants is not a risk-free procedure either15. Handel21 described the complications observed in patients who underwent secondary mastopexy and who had previously received breast implants. The presence of the implant causes anatomical and functional changes in the adjacent tissues, reduces the local blood supply, and thus leads to potential complications when using conventional mastopexy techniques.
A review of the literature reveals no consensus on the best technique for one-stage mastopexy with breast augmentation, nor regarding which procedure is more worthwhile the 1-stage or 2-stage. Moreover, with an increasing number of patients seeking treatment for breast ptosis with hypomastia, further studies are necessary to confirm the efficacy and safety of the various surgical techniques.
The aim of this study is to describe the surgical techniques for breast augmentation in patients with breast ptosis, as well as the incidence of complications and surgical revisions.
METHODS
We conducted a retrospective chart review of 38 patients who underwent one-stage mastopexy with breast augmentation between June 2005 and May 2010. The minimum postoperative follow-up period was 6 months. Of these 38 patients, we excluded those who underwent a secondary procedure (n = 2), those who had undergone previous mastopexy (n = 3), and those who had previously received a breast implant (n = 4). Patients who underwent mastopexy with breast augmentation combined with tumor resection, which was performed by the breast surgery teams, were also excluded (n = 2). Thus, 27 patients were included in this study, and their charts and photographic records were reviewed.
The surgical technique consisted of a mastopexy, resulting in an inverted-T-shaped scar, using the superomedial pedicle for the nipple-areola complex (NAC), combined with breast implant placement in a subglandular position. All surgical procedures were performed in hospitals, under general anesthesia. The breast implants that were used (Mentor Corporation) were round, high-profile, and had a textured surface.
The surgical marking was made in the immediate preoperative period in the hospital room, with the patient in a sitting position, and with the upper limbs aligned with the lateral side of the trunk. The midline was marked from the suprasternal notch to the umbilical scar, and a line was drawn along the breast from a point on the clavicle situated 6 cm from the suprasternal notch, corresponding to the meridian of the breast. The Pitanguy point (point A), corresponding to the upper border of the new areola, was marked on this latter line, on a point corresponding to the anterior projection of the mammary fold. The amount of skin to be resected was estimated using the pinch test. Two lines of approximately 11 cm were drawn from point A. From the end of these lines, 2 other lines were drawn that joined these points to the medial and lateral borders of the breast, in the inframammary fold.
The surgery was performed with the patient in the supine position, with the upper limbs in abduction at shoulder level, at 90º to the thorax. An incision was made in the inframammary fold until the muscular fascia was reached (Figure 1). A dissection was performed in the subglandular plane, using an electric scalpel, to approximately 4 cm above point A (Figure 2). The areola was then marked using an areola cutter, followed by decortication of a triangular area whose apexes were the point A and the 2 intersections of the previously drawn lines, with preservation of the areola (Figure 3).
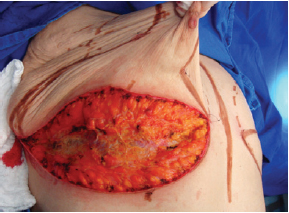
Figure 1 - Incision in the inframammary fold until the muscle fascia is reached.
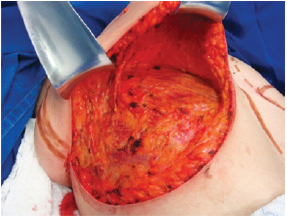
Figure 2 - Subglandular undermining until 4 cm above point A.
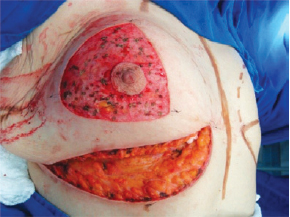
Figure 3 - Decortication of a triangle with its apexes at point A and at the intersection of the drawn lines.
Next, the superomedial pedicle for the NAC was marked and dissected (Figures 4 and 5). The mammary tissue corresponding to the lower pole and central portion was resected, with preservation of the pedicle and NAC (Figure 6).
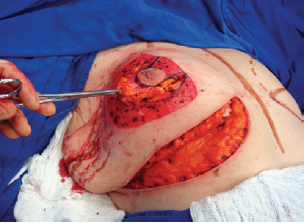
Figure 4 - Dissection of the superomedial pedicle for the nipple-areolar complex.
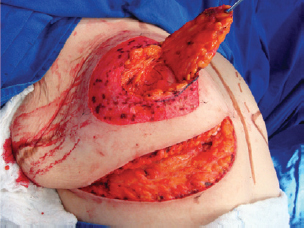
Figure 5 - Elevated superomedial pedicle.
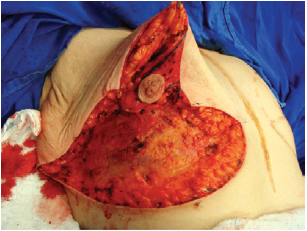
Figure 6 - Resected lower and central poles.
The NAC was elevated to the previously marked point A position (Figure 7), and the lateral and medial portions were joined to the center and sutured (Figure 8). Following completion of the vertical suture, the breast implant was placed immediately below the mammary tissue, and above the muscle fascia (Figure 9).
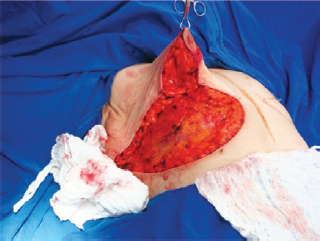
Figure 7 - Nipple-areolar complex positioned at point A.
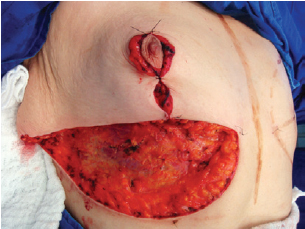
Figure 8 - Lateral and medial aspects joined to the center
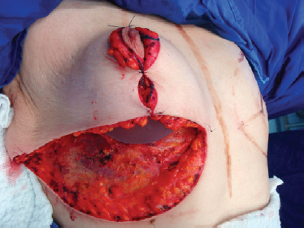
Figure 9 - Implant positioned under the mammary tissue.
The suture of the horizontal incision in the inframammary fold led to complete coverage of the implant (Figures 10 and 11).
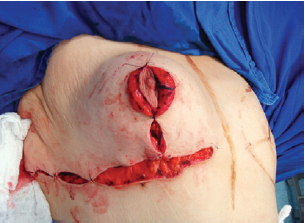
Figure 10 - Closure of the inframammary fold, covering the implant completely.
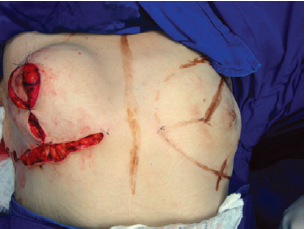
Figure 11 - Appearance of the breast at the end of the procedure.
A suction drain was placed along the lower edge of the implant, and exited through a small separate incision. All patients received prophylactic antibiotic therapy with a second-generation cephalosporin that was initiated upon anesthesia induction and maintained until 7 days postoperatively.
RESULTS
The average age of the 27 study patients was 36.7 years, and ranged from 23 to 54 years. Four (14.8%) patients were formerly morbidly obese, and had previously undergone bariatric surgery. Eight (29.6%) patients had a history of smoking, but all had ceased smoking at least 1 year before the procedure. Five (18.5%) patients were being treated for hypertension. One (3.7%) patient had a history of asthma, with regular use of corticosteroids and short-acting beta-agonists. There was no history of diabetes among the study patients. The average body mass index (BMI) was 24.4 kg/m2, with a range of 19.5 kg/m2 to 28.6 kg/m2. Three (11.1%) patients had a BMI above 25 kg/m2. Of the 27 patients, 18 (66.7%) had a history of pregnancy; their last pregnancies had occurred more than 1 year before the procedure.
The postoperative follow-up period varied between 6 months and 5 years (average, 1.8 years) (Figures 12 to 14).
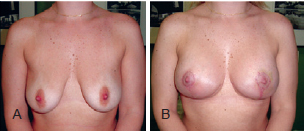
Figure 12 - Patient A. In A, preoperative appearance. In B, appearance 6 months after the procedure.
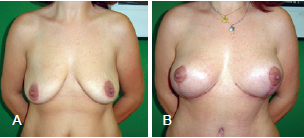
Figure 13 - Patient B. In A, preoperative appearance. In B, appearance 6 months after the procedure.
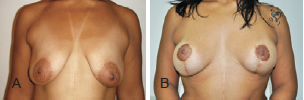
Figure 14 - Patient C. In A, preoperative appearance. In B, appearance 16 months after the procedure.
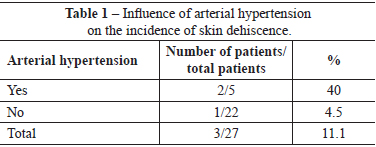
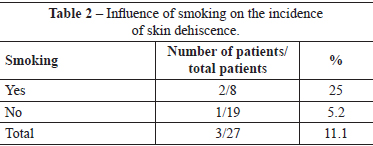
There were no immediate complications requiring early reoperations. One (3.7%) patient developed late hematoma on the 30th day postoperatively, as a result of a fall. This patient, who did not have a history of morbidity, underwent drainage of the hematoma in a surgery center, and ligation of the perforating arterial branch of the pectoralis major muscle, which was actively bleeding. Three (11.1%) patients had slight suture dehiscence in the junction of the inverted-T scar, which resolved spontaneously after clinical treatment by local cleaning and daily wound dressing. Of these patients, 1 had a history of arterial hypertension, 1 had a history of arterial hypertension and smoking, and 1 had a history of smoking. There were no cases of total or partial necrosis of the NAC (Tables 1 and 2).
All patients were discharged within 1 day of the procedure. The suction drain was retained for 3 to 5 days (average, 3.8 days).
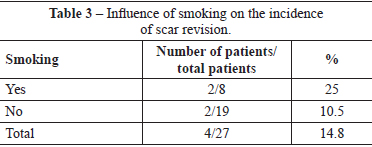
Four (14.8%) patients underwent scar revision. Of these patients, 2 patients had a history of smoking, and 2 patients did not have any comorbidities. Two of these patients belonged to the group of patients with slight suture dehiscence (Table 3).
One (3.7%) patient developed capsular contracture that was found 1 year postoperatively; she underwent reoperations including partial capsulectomy, breast implant replacement, and placement of the implant in the submuscular position. Two (7.4%) patients underwent reoperations to replace the breast implants for larger-sized ones, without changing the position of the implants or capsulectomy; these 2 patients belonged to the group of 4 patients who underwent one-stage scar revision and breast implant replacement. There were no complications in the group of formerly morbidly obese patients.
DISCUSSION
In some patients seeking plastic surgery for breast augmentation, breast implant placement alone does not ensure adequate treatment of the breast region. These cases include breast ptosis, breast asymmetries, tuberous breasts, and, more recently, breast alterations due to massive weight loss after bariatric surgery8,11. Frequently, patients who seek plastic surgery for breast augmentation are unaware of the need to correct breast ptosis. Attempts to correct breast ptosis with large-volume breast implants often yield unsatisfactory aesthetic results in the medium and long term3,5. Similarly, the aesthetic result of mastopexy techniques or reduction mammaplasty alone, without breast implant placement, is also often not satisfactory for the patient or the surgeon22.
The one-stage combination of mastopexy with breast augmentation has the advantage of facilitating aesthetic treatment of the breast by modifying it in several aspects simultaneously, such as volume, projection, shape, position, symmetry, elevation, and width of the NAC. However, this advantage can result in an increased complexity in the surgical approach, as the final aesthetic result is then dependent on all these variables4. Thus, careful and thorough planning of this procedure is fundamental. Moreover, the complications are potentially more serious when mastopexy is used in combination with breast augmentation3,4.
In the present study, a surgical technique for one-stage mastopexy combined with breast augmentation for the treatment of breast ptosis is described. The superomedial pedicle technique was used; it safely allows for extensive migration of the NAC and its vascularization by a pedicle, through anterior intercostal branches and branches of the internal thoracic vessels. In addition, this technique greatly preserves skin vascularization, because it does not involve the separation of the skin from the deep tissues23. The preoperative skin marking for mastopexy combined with breast augmentation takes into consideration the larger total breast volume as a result of the augmentation; thus, less skin tension is observed by the pinch test.
The placement of the breast implant in the subglandular position allows for better distribution of the tension under the mammary tissue that results from mastopexy. Thus, uniform projection throughout the tissues that cover the implant (skin and mammary tissue) is achieved. However, this positioning of the implant in mastopexy combined with breast augmentation results in a lesser amount of tissue available for covering the implant, compared to that obtained by submuscular positioning. In addition, it can reduce implant support, leading to recurrent ptosis, as well as downward displacement of the implant and breast pseudoptosis. On the other hand, submuscular placement of the implant can result in breast ptosis above the pectoralis major muscle and above the implant, thus creating a double-bubble appearance. In the present study, no recurrent breast ptosis or pseudoptosis were observed with the implants placed subglandularly. The rate of capsular contractures was 3.7%, which is in accordance with the available data.
As this is a surgical technique involving extensive tissue undermining and mobilization, the procedure is performed with careful attention to preservation of tissue integrity, skin and NAC vascularization, and thorough hemostasis. There was one case of late hematoma, which developed 30 days postoperatively, that was clearly associated with direct trauma in the region as a result of a fall. This patient exhibited active bleeding from the medial perforating arterial branch, in the medial border of the capsule. The bleeding was most likely caused by the traumatic mobilization of the implant to the medial region, with the rupture of the medial perforating arterial branch. In this case, the procedure involved surgical exploration of the hematoma, to ensure intracapsular and peri-implant asepsis. In addition, performing the procedure in a surgical center allowed for correct manipulation of the implant, the preservation of its integrity, and adequate localization and ligation of the blood vessel responsible for the bleeding.
The blood supply of the skin and NAC is an important focus of attention in mastoplasty24. In the case of mastopexy combined with breast augmentation, it is a critical aspect that can be potentially disastrous if tissues that protect the implant are not preserved3. When extensive tissue undermining and mobilization techniques are used, many nutritive vessels are severed, and blood supply becomes restricted. Consequently, there is a reduction in the nutritive supply to the tissue; tissue survival and healing depends on the resistance to the nutritional shortage. In the present study, there was no case of partial or total necrosis of the NAC. The use of the superomedial pedicle facilitates the safe migration of the NAC to point A, with good blood supply from the large vessels, and without significant anatomical changes. However, 3 (11.1%) patients had small areas of skin necrosis in the inverted-T junction that indicated the combination of terminal vascularization in that outermost area of skin with excessive closure tension, either due to the use of an implant that was too large, or due to inappropriate marking that led to skin tension. Exposure of the implant was not observed in these 3 patients, and there was complete epithelialization of the resulting wound within 3 weeks after the procedure. Two patients were hypertensive (40% of the patients with skin dehiscence), and 2 were smokers (25% of the patients with skin dehiscence). In this study, arterial hypertension caused a nine-fold increase in the risk of skin dehiscence. Furthermore, smoking was associated with a four-fold increased risk of this complication.
Five (18.5%) patients underwent surgical revision. The indications for revision were hypertrophic scars in 4 patients and capsular contracture in 1 patient. Of the patients with hypertrophic scars, 2 were ex-smokers. Thus, 25% of the patients who were smokers underwent scar revision. The patient who underwent replacement of the breast implant as a result of capsular contracture only had a past history of bronchial asthma; in this patient, the episodes of bronchospasm were treated with beta-agonist and corticosteroid administration. This patient did not have an episode of bronchospasm in the perioperative period or in the year after the primary surgery. Thus, smokers, and even ex-smokers, are susceptible to scar changes, such as dyschromia, scar widening, hypertrophy, and atrophy. In the present study, a history of smoking resulted in a twofold increase in the rate of scar revision. This increase in the incidence of unsatisfactory scars suggests a negative influence of smoking on the skin tension of the breast subject to implant insertion combined with mastopexy.
Table 4 presents data regarding complications observed in the study.
CONCLUSIONS
This study describes the one-stage mastopexy technique using the superomedial pedicle for the NAC, combined with a breast implant placed in the subglandular position. This technique was effective for the treatment of patients with breast ptosis who desired breast augmentation and correction of ptosis. The incidence of complications was in accordance with the available data. Systemic arterial hypertension and a history of smoking increased the risk of minor suture dehiscence and scar revision.
REFERENCES
1. Regnault P. The hypoplastic and ptotic breast: a combined operation with prosthetic augmentation. Plast Reconstr Surg. 1966;37(1):31-7.
2. Gonzalez-Ulloa M. Correction of hypotrophy of the breast by means of exogenous material. Plast Reconstr Surg Transplant Bull. 1960;25:15-26.
3. Spear S. Augmentation/mastopexy: "surgeon, beware". Plast Reconstr Surg. 2003;112(3):905-6.
4. Spear SL, Low M, Ducic I. Revision augmentation mastopexy: indications, operations, and outcomes. Ann Plast Surg. 2003;51(6):540-6.
5. Spear SL, Boehmler JHT, Clemens MW. Augmentation/mastopexy: a 3-year review of a single surgeons practice. Plast Reconstr Surg. 2006;118(7 Suppl):136S-47S.
6. Nahai F, Fisher J, Maxwell PG, Mills DC 2nd. Augmentation mastopexy: to stage or not. Aesthet Surg J. 2007;27(3):297-305.
7. Pitanguy I, Carreirão S, Mazzarone F, Fróes LB, Cló TT. Mastopexia associada à inclusão de prótese. Rev Bras Cir Plást. 1991;81(2):101-11.
8. Carramaschi FR, Tanaka MP. Mastopexia associada a inclusão de prótese mamária. Rev Bras Cir Plást. 2003;18(1):25-36.
9. Stevens WG, Stoker DA, Freeman ME, Quardt SM, Hirsch EM, Cohen R. Is one-stage breast augmentation with mastopexy safe and effective? A review of 186 primary cases. Aesthet Surg J. 2006;26(6):674-81.
10. Stevens WG, Freeman ME, Stoker DA, Quardt SM, Cohen R, Hirsch EM. One-stage mastopexy with breast augmentation: a review of 321 patients. Plast Reconstr Surg. 2007;120(6):1674-9.
11. Gomes RS. Mastopexia com retalho de pedículo superior e implante de silicone. Rev Bras Cir Plást. 2008;23(4):241-7.
12. Karacaoglu E. Single stage augmentation mastopexy: a novel technique using autologous dermal graft. Ann Plast Surg. 2009;63(6):600-4.
13. Lindsey JT. Importance of the periareolar approach in the augmentation of the ptotic breast. Ann Plast Surg. 2002;48(5):460-2.
14. Cannon CL 3rd, Lindsey JT. Conservative augmentation with periareolar mastopexy reduces complications and treats a variety of breast types: a 5-year retrospective review of 100 consecutive patients. Ann Plast Surg. 2010;64(5):516-21.
15. Seify H, Ismail K, Evans G. Primary augmentation/mastopexy using large implants: is it a safe technique? A 4-year single surgeon review. Plast Reconstr Surg. 2010;126(Suppl. 4):67.
16. Kwei S, Borud LJ, Lee BT. Mastopexy with autologous augmentation after massive weight loss: the intercostal artery perforator (ICAP) flap. Ann Plast Surg. 2006;57(4):361-5.
17. Rubin JP, Khachi G. Mastopexy after massive weight loss: dermal suspension and selective auto-augmentation. Clin Plast Surg. 2008;35(1):123-9.
18. Colwell AS, Driscoll D, Breuing KH. Mastopexy techniques after massive weight loss: an algorithmic approach and review of the literature. Ann Plast Surg. 2009;63(1):28-33.
19. Don Parsa F, Brickman M, Parsa AA. Augmentation/mastopexy. Plast Reconstr Surg. 2005;115(5):1428-9.
20. Elliott LF. Circumareolar mastopexy with augmentation. Clin Plast Surg. 2002;29(3):337-47.
21. Handel N. Secondary mastopexy in the augmented patient: a recipe for disaster. Plast Reconstr Surg. 2006;118(7 Suppl):152S-63S.
22. Ferreira MC. Evaluation of results in aesthetic plastic surgery: preliminary observations on mammaplasty. Plast Reconstr Surg. 2000;106(7):1630-5.
23. Orlando JC, Guthrie RH Jr. The superomedial dermal pedicle for nipple transposition. Br J Plast Surg. 1975;28(1):42-5.
24. Silveira-Neto E. Mastoplastia redutora setorial com pedículo areolar interno. XIII Congresso Brasileiro de Cirurgia Plástica; 1976; Porto Alegre, RS, Brasil.
1. Master in plastic surgery, full member of the Sociedade Brasileira de Cirurgia Plástica (SBCP), assistant physician at the Plastic Surgery Department of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), São Paulo, SP, Brazil.
2. Plastic surgeon, associate member of the SBCP, preceptor physician at the Plastic Surgery and Burns Department of the HCFMUSP, São Paulo, SP, Brazil.
3. Professor, plastic surgeon, full member of the SBCP, associate professor at the Plastic Surgery and Burns Department of the HCFMUSP, São Paulo, SP, Brazil.
4. Full professor at the Faculdade de Medicina of the Universidade de São Paulo, plastic surgeon, full member of the SBCP, head of the Divisão de Cirurgia Plástica e Queimaduras do HCFMUSP, São Paulo, SP, Brazil.
Correspondence to:
Alexandre Wada
Av. Dr. Enéas Carvalho de Aguiar, 255 - 8º andar - sala 8.128 - Cerqueira César
São Paulo, SP, Brazil CEP 05403-000
E-mail: alexandrewada@gmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: September 2, 2012
Article accepted: October 15, 2012
This study was performed at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (Hospital das Clinicas, Faculty of Medicine, University of São Paulo), São Paulo, SP, Brazil.
The study was presented at the 48th Brazilian Congress of Plastic Surgery in order to obtain the title of full member of the Sociedade Brasileira de Cirurgia Plástica.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license