

Original Article - Year 2025 - Volume 40Issue 1
Series of BIA-ALCL Cases in the Federal District, Brazil
Série de casos de BIA-ALCL no Distrito Federal, Brasil
ABSTRACT
Introduction Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare type of non-Hodgkin lymphoma manifesting in women with breast implants.
Objective To analyze cases of BIA-ALCL treated by plastic surgeons in the Federal District, Brazil. Materials and Methods We conducted a retrospective observational study with patients diagnosed with BIA-ALCL, analyzing demographic data, implant features, treatment, and prognosis.
Results The mean age at diagnosis was of 45 years, and the mean time of implantation was of 8.3 years. All cases involved textured polyurethane implants. The most frequent initial symptom was seroma, and periprosthetic fluid aspiration with cytopathological analysis was crucial for the diagnosis. The treatment consisted of implant removal and capsulectomy in all cases. No patient required adjuvant chemotherapy or radiation therapy. The overall prognosis was favorable.
Discussion The findings corroborate the epidemiological and clinical characteristics of BIA-ALCL described in the literature. Early symptom recognition and timely diagnosis are critical. Definitive diagnosis requires peri-implant fluid aspiration. Treatment with immediate implant removal and total capsulectomy was effective. We emphasize the significance of regular medical surveillance for women with breast implants.
Conclusion Breast implant-associated anaplastic large cell lymphoma is a rare but potentially-severe disease. Early diagnosis and appropriate treatment are essential for a good prognosis. Further studies with a larger number of patients are required to confirm the findings and deepen the knowledge about BIA-ALCL.
Keywords: Brazil; breast implants; implant capsular contracture; lymphoma, large-cell, anaplastic; polyurethane; seroma
RESUMO
Introdução O linfoma anaplásico de grandes células associado a implante mamário (breast implant-associated anaplastic large cell lymphoma, BIA-ALCL, em inglês) é um tipo raro de linfoma não Hodgkin que se manifesta em mulheres com implantes mamários. Objetivo Analisar casos de BIA-ALCL tratados por cirurgiões plásticos no Distrito Federal.
Materiais e Métodos Conduziu-se um estudo observacional retrospectivo com pacientes diagnosticadas com BIA-ALCL, em que foram analisados os dados demográficos, as características dos implantes, o tratamento realizado e o prognóstico das pacientes. Resultados A idade média ao diagnóstico foi de 45 anos, e o tempo médio de implante foi de 8,3 anos. Todos os casos analisados envolviam implantes de poliuretano texturizados. Seroma foi o sintoma inicial mais frequente, e a punção do líquido periprotésico com análise citopatológica foi crucial para o diagnóstico. O tratamento consistiu na retirada dos implantes e em capsulectomia em todos os casos. Nenhuma paciente necessitou de quimioterapia ou de radioterapia adjuvante. O prognóstico geral foi favorável.
Discussão Os achados corroboram as características epidemiológicas e clínicas do BIA-ALCL descritas na literatura. É de extrema importância o reconhecimento precoce dos sintomas e o diagnóstico oportuno, sendo a punção do líquido peri-implantar uma ferramenta essencial para o diagnóstico definitivo. O tratamento com retirada imediata dos implantes e capsulectomia total foi eficaz. Ressalta-se a importância da vigilância médica regular para mulheres com implantes mamários.
Conclusão O BIA-ALCL é uma doença rara, mas potencialmente grave. O diagnóstico precoce e o tratamento adequado são essenciais para um bom prognóstico. Mais estudos comum número maior de pacientes são necessários para confirmar os achados e aprofundar o conhecimento sobre o BIA-ALCL.
Palavras-chave: Brasil; contratura capsular em implantes; implantes de mama; linfoma anaplásico de células grandes; poliuretano; seroma
Introduction
Approximately 35 million women worldwide have breast implants. According to estimates, more than two million Brazilian women have breast implants, making Brazil the second-largest market for breast implants in the world.
Breast implants with silicone prostheses started in 1962. Since then, several studies have aimed to uncover and analyze the impacts of these substances on the body.
In 2011, the United States Food and Drug Administration (FDA) identified a potential link between breast implants and an uncommon form of non-Hodgkin lymphoma called anaplastic large cell lymphoma (ALCL). In 2016, the World Health Organization (WHO) guidelines ratified the disease as breast implant-associated anaplastic large cell lymphoma (BIAALCL).1
A rare type of lymphoma, BIA-ALCL has gained prominence in the last 2 decades due to the increased number of cases reported. The exact incidence of BIA-ALCL remains unknown, but it is estimated to be of approximately 1 case per 30 thousand women with implants per year, with an average time for disease development after implantation of 10 years.2
According to regulatory agencies, the risk of disease development is associated with textured-surface breast implants.3 Breast implant-associated anaplastic large cell lymphoma may occur in the seroma cavity or involve the pericapsular fibrous tissue. Most patients present with peri-implant effusion and, less frequently, with a mass. Diagnosis relies on aspirating the peri-implant effusion and confirming CD30 positivity in the sampling cells. However, diagnostic confirmation can be a challenge. Combining the presence of characteristic cells with the results of flow cytometry and immunohistochemistry can aid in obtaining an accurate diagnosis.4 Most patients have an excellent prognosis after complete capsule removal and surgical implantation of a prosthesis with negative margins.5
Objective
The present study aimed to analyze cases of BIA-ALCL treated by plastic surgeons in the Federal District, Brazil.
Materials and Methods
The current retrospective observational study started with data collection from medical record analysis after obtaining informed consent from plastic surgeons and patients in Brasília, Federal District, Brazil, in 2024. We used demographic data to analyze implant features (type, texture and year of implantation), postdiagnosis treatment, and prognosis. The Ethics Committee of Hospital Daher Lago Sul approved the study.
Results
Demographic Data and Implant Features The average age at diagnosis of BIA-ALCL was 45 years, while the average time of implant use was 8.3 years, which suggests that the disease may manifest late after placement.
All cases involved polyurethane implants, indicating a potential association between this type of prosthesis and the development of BIA-ALCL. Among our patients, three presented with comorbidities (one had rheumatoid arthritis, the second presented with insulin resistance, and the third had breast cancer) (►Table 1).
| Cases | Initials | Birth date | Comorbidities | Date of implant placement | Implant features | Date of surgery for removal |
|---|---|---|---|---|---|---|
| 1 | GCLAO | March 25, 1976 (44 years old on removal) | None | 2015 | Polyurethane, 330 mL | May 14, 2020 (5 years after implantation) |
| 2 | VCR | August 04, 1971 (52 years old on removal) | Breast neoplasm in 2015 (same date of implant placement) | 2015 | Polyurethane, 305 mL | June 28, 2023 (8 years after implantation) |
| 3 | FSSFC | December 30, 1981 (41 years old on removal) | None | 2015 | Polyurethane, 280 mL | October 13, 2023 (8 years after implantation) |
| 4 | AJBSB | April 12, 1979 (44 years old on removal) | Insulin resistance | 2013 | Polyurethane, 435 mL | April 2023 (10 years after implantation) |
| 5 | KLBCGB | December 27, 1975 (48 years old on removal) | Rheumatoid arthritis | 2011 | Polyurethane, 315 mL | February 2023 (12 years after implantation) |
| 6 | DCM | December 10, 1979 (41 years old on removal) | None | 2014 | Polyurethane, 330 mL | October 04, 2021 (7 years after implantation) |
Symptomatology and Diagnosis
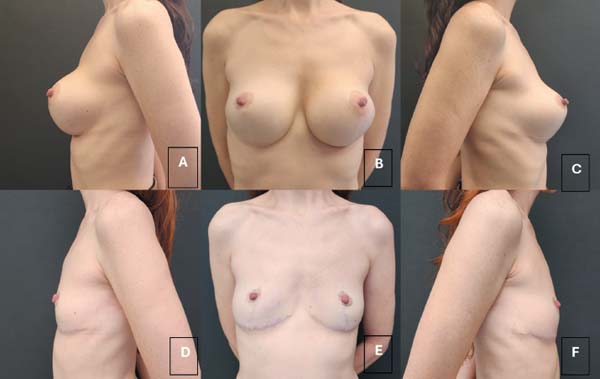
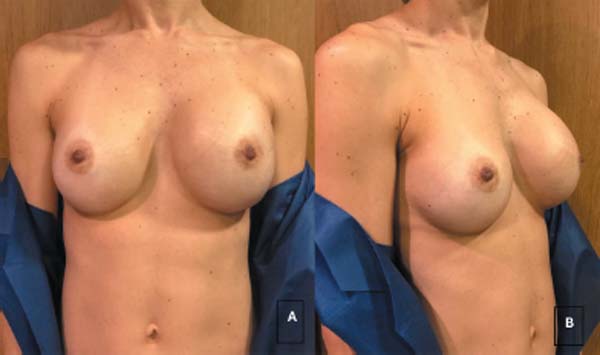
The most common initial symptom was seroma (►Fig.1), followed by intense mastalgia and capsular contracture in one case. In all cases, periprosthetic fluid aspiration with cytopathological analysis was crucial for the diagnosis of BIA-ALCL (►Table 2).
| Cases | Initials | Symptoms | Laboratory |
|---|---|---|---|
| 1 | GCLAO | Sudden increase in left breast volume, with little pain. Seroma puncture performed (►Fig. 1). | Lab/Diagnose |
| 2 | VCR | Slight increase in left breast volume. Fluid puncture performed. | Diagnose |
| 3 | FSSFC | Breast augmentation and seroma in March 2023. The third puncture revealed BIA-ALCL. | Diagnose |
| 4 | AJBSB | Painless seroma in the left breast in January 2023. Puncture with BIA-ALCL diagnosis | Lâmina |
| 5 | KLBCGB | Intense mastalgia, capsular contracture, December 2022 | Diagnose |
| 6 | DCM | Itching, night sweats, skin lesions | Diagnose |
Treatment and Prognosis
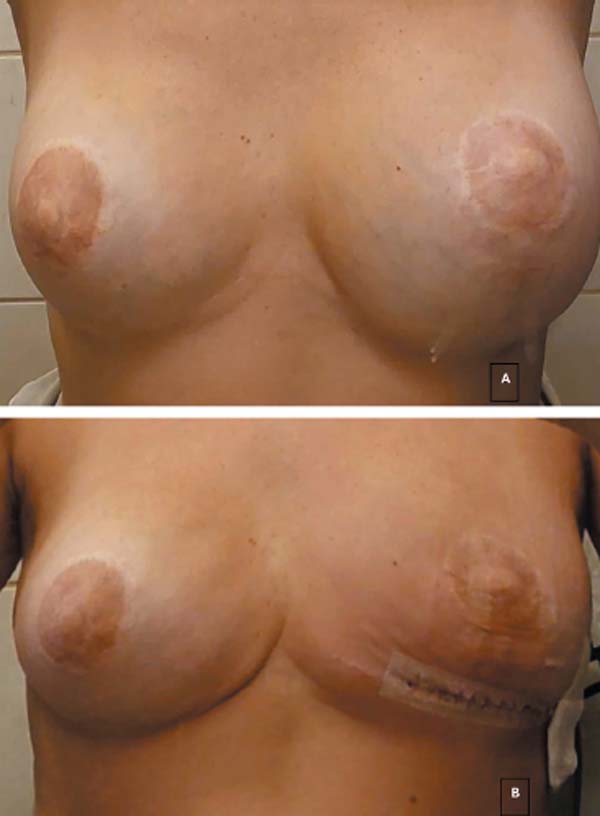
All cases underwent breast implant removal and capsulectomy. This approach represented the primary treatment for BIA-ALCL in the current study (►Figs. 2 and 3). None of the patients required adjuvant chemotherapy nor radiation therapy due to the absence of distant disease. One patient opted for new microtextured breast implants 8 months after removing the affected implants.
The general prognosis was favorable, with all patients showing good progress after treatment (►Table 3).
| Cases | Initials | Surgery | Supplementary treatment required | Notes | Prognosis |
|---|---|---|---|---|---|
| 1 | GCLAO | Implant removal and en bloc capsulectomy with implant exchange. | No | No abnormalities on positron emission tomography– computed tomography | Good |
| 2 | VCR | Prosthesis removal and en bloc capsulectomy on the left side, without placement of new implants (►Fig. 2). After the anatomopathological report showing no capsular disease, a new implant was placed on the left side, and the right-sided implant was replaced on December 11, 2023. | No | Good | |
| 3 | FSSFC | Implant removal with en bloc capsulectomy, breast reconstruction with the remaining tissue, with no fat grafting nor new implant placement (►Fig. 3). | No | Good | |
| 4 | AJBSB | Explantation, capsulectomy, and adhesion sutures between the breast tissue and the pectoralis major muscle. No fat grafting or implant placement at the same time. | No | An oncology follow-up ruled out distant disease, enabling a new implant 8 months after the explant (placement of microtextured implants in the submuscular plane) | Good |
| 5 | KLBCGB | Explantation, capsulectomy, and adhesion sutures between the breast tissue and the pectoralis major muscle. No fat grafting nor implant placement at the same time. | No | Cytological and anatomopathological examinations concluded that the disease was confined to the fluid. Follow-up with oncology did not indicate adjuvant treatment. | Good |
| 6 | DCM | Explantation, lymphadenectomy, and breast reconstruction with mastopexy without implants. | No | Good |
Discussion
Our data corroborates the epidemiological and clinical characteristics described in the literature about BIA-ALCL. The mean age at diagnosis, the latency time after implant placement, and the predominance of polyurethane implants are consistent with other studies.
The present study also highlights the significance of early symptom recognition, especially seroma, for the timely diagnosis of BIA-ALCL. Diagnostic aspiration of peri-implant fluid is essential for the definitive diagnosis.6
Guidelines for Collection and Packaging of Late Periprosthetic Breast Effusions
Percutaneous puncture: Collect 10 to 50 mL of effusion (use ultrasound guidance for small collections).
Avoid prior drainages, as they reduce the tumor load for later analysis.
If taking the specimen IMMEDIATELY to the laboratory, there is no need to add a fixation medium.
If it takes more than 1 hour to get to the laboratory, add absolute alcohol in a 50/50 ratio to the fluid, or 70% alcohol in a 2/3 ratio of alcohol and 1/3 of fluid, or formalin (10%, buffered) in a 50/50 ratio.
Report on the medical order the fixative used, the time of the prosthesis, the effusion time, the condition, type, and texture of the prosthesis, how many milliliters were punctured, the characteristics of the fresh fluid, whether there was an associated palpable mass or palpable axillary lymph node, imaging data (if any), and other relevant clinical findings.
Request a cytopathological examination, preparation of a cell block, and immunocytochemical study with CD30 cell count (differential for BIA-ALCL).
It is possible to store 3.0 to 4.0 mL of the fluid in a dry cerebrospinal fluid tube (requested in advance from the clinical laboratory) for flow cytometry for hematologic neoplasms (CD30) – order it separately.
Note: Puncture and cytological diagnosis should precede capsulectomy/en bloc explantation whenever possible. This enables the postdiagnostic optimization of staging (positron emission tomography–computed tomography; PET-CT) and surgical planning.
During surgery, after en bloc explantation, puncture the effusion for adequate fluid preservation, enabling diagnostic confirmation and correct staging.
Treatment with immediate implant removal and total capsulectomy has been proven to be effective in controlling the disease. Most patients did not require adjuvant chemotherapy or radiation therapy.7
Reimplantation of breast implants after BIA-ALCL treatment is an individualized decision that should be made cautiously and occur under strict medical supervision. No patient received fat grafting to improve the esthetic outcome. The overall prognosis was favorable, with all patients showing good progress after treatment. This highlights the significance of early diagnosis and adequate management.
Additional Considerations
The present study highlights the significance of regular medical monitoring for women with breast implants, especially 10 years after implantation.
Further studies with a larger number of cases are needed to confirm the association between polyurethane implants and BIA-ALCL and to refine treatment and follow-up strategies.
Recommendations
Increased awareness of BIA-ALCL among the medical community and patients.
Implementation of standardized protocols for BIA-ALCL diagnosis and treatment.
Prospective data collection in multicenter studies to expand knowledge about the disease and identify potential risk factors.
Conclusion
It is worth emphasizing that the current study presents a low number of cases, which limit the generalizability of the conclusions. Further studies with larger samples are needed to confirm the findings and deepen our understanding of BIA-ALCL.
Despite its limitations, the present study provides valuable information about BIA-ALCL, contributing to improving diagnosis, treatment, and monitoring of this rare disease. Disseminating knowledge about BIA-ALCL is essential for proper patient care and to prevent new cases.
REFERENCES
1. DB Diagnósticos. Linfoma anaplásico de grandes células (ALCL) associado ao implante mamário (BIA). São José dos Pinhais, PR: DB Diagnósticos. Disponível em: https://www.diagnosticosdobrasil.com.br/uploads/materiais/2021/05/db-patol-lamina-linfoma-anaplasico-com-logo.pdf
2. Real DSS, Resendes BS. Linfoma anaplásico de grandes células relacionado ao implante mamário: revisão sistemática da literatura. Rev Bras Cir Plást 2019;34(04):531–538. Doi: 10.5935/2177-1235.2019RBCP0234
3. Esteves RC, Silva JDFd. BIA-ALCL: Por que alguns implantes mamários de superfície texturizada estão sendo retirados do mercado? Rev Inovativa 2020;7(30). Disponível em: https://www.gov.br/int/pt-br/assuntos/revista-inovativa/edicoes/edicao-30/bia-alcl-por-que-alguns-implantes-mamarios-de-superficie-texturizada-estao-sendo-retirados-do-mercado
4. Jaffe ES, Ashar BS, Clemens MW, Feldman AL, Gaulard P, Miranda RN, et al. Best Practices Guideline for the Pathologic Diagnosis of Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2020;38(10):1102–1111. Doi: 10.1200/JCO.19. 02778
5. De-Azambuja AP, Groth AK, Jung J, Gevert F, Nabhan SK. Linfoma anaplásico de células grandes associado a implante mamários: um desafio diagnóstico. Rev Bras Cir Plást 2020;35(01):118–120. Doi: 10.5935/2177-1235.2020RBCP0019
6. Longo B, Di Napoli A, Curigliano G, Veronesi P, Pileri S, Martelli M, et al. Clinical recommendations for diagnosis and treatment according to current updated knowledge on BIA-ALCL. Breast 2022;66(02):332–341. Doi: 10.1016/j.breast.2022.11.009
7. Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J 2019;39(Suppl 1):S3–S13. Doi: 10.1093/asj/sjy331
1. Hospital Daher Lago Sul, Brasília, DF, Brazil
Address for correspondence Lana Gabriela de Sousa Silva, Hospital Daher Lago Sul, Área Especial 04, Lotes I/J, apto. 206, Torre 3, Residencial Sports Club, Guará 2, Brasília, DF, 71070–904, Brazil (e-mail: lanagabriela@hotmail.com; lanagabrielass@gmail.com).
Article received: December 18, 2024.
Article accepted: March 20, 2025.
Conflict of Interests
The authors have no conflict of interests to declare.
CAAE 83000324.9.0000.0257.





 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter