INTRODUCTION
Polymethylmethacrylate (PMMA) is a permanent filler material (PP), consisting of
small spheres suspended in different vehicles such as hyaluronic acid, ascorbic
acid, bovine collagen, polyethylene glycol and magnesium carboxygluconate
hydrolactic acid. Its use on the face, like any other filler, can lead to
complications. The growing demand for non-surgical procedures for rejuvenation,
stimulating collagen formation, and increasing facial volume, including the
injection of this product, has brought with it an increase in cases of
complications. The diagnosis of complications related to this material is
normally clinical, obtained through the patient’s history and evaluation and
imaging tests such as ultrasound, magnetic resonance imaging, and computed
tomography1.
Complications related to the use of PMMA on the face can occur immediately,
early, late, or many years after its initial application. The main complications
are represented by the formation of granulomas due to a foreign body or nodules,
leading to deformities, inflammatory reaction, infection, intermittent edema,
pigmentation, neovascularization, functional limitation or deformity of
structures such as the mouth or eyelids, tearing, fistulas, blindness, and
necrosis, among others2.
Although most of the medical literature addresses the different complications and
their treatments, there are few studies showing the relationship between these
problems and variables such as gender, age group, number of product
applications, and the relationship between these variables and the time before
complications appear. This fact shows the lack of a broader understanding
regarding complications and other factors that lead to a better understanding,
prevention, and treatment of these problems.
The moment of emergence, anatomical region, and type of manifestation remain
little known and unpredictable. It is not uncommon for patients to experience
problems many years after the initial injection. Thus, multiple factors will
determine the most appropriate therapy or set of therapies for each case.
The main treatments proposed for complications related to PMMA injection into the
face are the application of medication and surgical approach. Intralesional
corticosteroid injection is the most commonly used resource. Most reports of
complications related to PP are based on retrospective studies, reports, or case
series. This leads to very variable estimates of complications. Granuloma
formation is one of the most reported in the medical literature3.
OBJECTIVE
We carried out a retrospective case series study of patients with pre-established
complications related to the application of PMMA to the face. Two hundred and
nine patients were evaluated. The main objective was to determine the median
time for complications to occur. The secondary objectives were to identify the
most compromised areas, the types of complications, and the treatments most
performed. Furthermore, it was determined whether the number of applications was
related to the earliest onset of complications, whether there was a difference
in the time of occurrence of complications according to gender, and whether
there was a difference in the time of occurrence of complications according to
age group.
METHOD
In a retrospective case series review, 209 individuals with complications after
PMMA injection in the face and who sought treatment in the corresponding
author’s private practice from January 2000 to June 2021 were reviewed. The
electronic medical records were searched using the “Personal Med (TOTVS)”
database software for the following keywords: PMMA, polimetilmetacrilato,
Artecoll, Metacril, Bioplastia, Biossimetric, Metacrilato, Newplastic, Artefill,
LinneaFace, Bioplastia, Arteplast, Bellafill. From then on, only patients who
had any complications were included in the study.
The exclusion criteria were: individuals whose medical records had one or more of
these keywords, but the cause of the consultation was not related to the
complication resulting from the previous application of PMMA. Patients were also
excluded from the study if their clinical history was unclear as to whether the
product actually applied was PMMA, if they had applied at least more than one
product, or when the material was injected by non-physicians.
Complications were diagnosed through clinical history, imaging tests such as
ultrasound, computed tomography or magnetic resonance imaging, photographic and
histological analysis, and clinical examination. Based on the date of the
initial application and the moment the complication appeared, the time elapsed
until the complication appeared was calculated.
The following information was collected in the review: sex, age, number of PMMA
injections performed on the patient when the application was performed, time
interval until the complication appeared, type of complication, the region
affected by the complication, and treatment performed. Gaussian quantitative
data were described by mean and standard deviation. In breaking distributional
assumptions, we opted for median and percentiles. Categorical data were
expressed as counts and percentages. To describe the occurrence of events over
time, Kaplan-Meier curves were created with a comparison between groups using
the log-rank test.
The groups were separated by sex and age range of up to 50 years and over 50
years. Values of p<0.05 were considered statistically
significant. Data analysis was performed using the IBM-SPSS version 25.0
program. As this is a descriptive study, a sample size was not calculated to
test hypotheses. 209 individuals who met all inclusion criteria were included.
This study was approved by the PUCRS Research Ethics Committee, under number
CAAE 26778719.3.0000.5336, and approval opinion number 3,786,448.
RESULTS
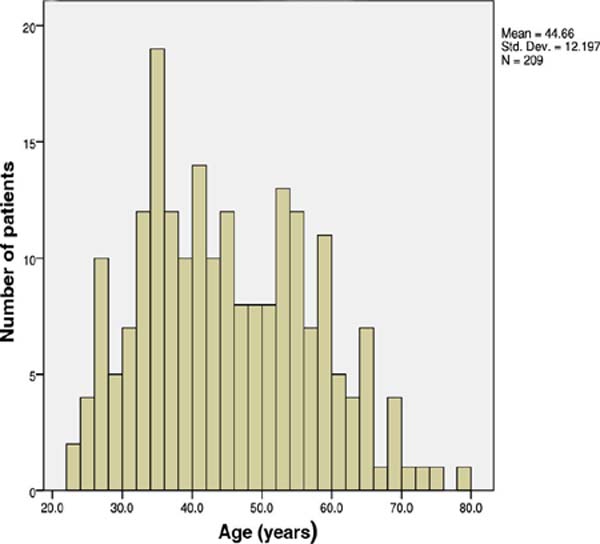
The mean age was (±SD) of 44.6 (±12.2) years. The median was 43. The youngest
individual was 23 years old and the oldest was 79 years old. Of these, 172 were
women (82.3%) and 37 men (17.7%) (Figure 1).
Figure 1 - Histogram representing age distribution. The average age of the
patients analyzed was 44.6 years. The age with the highest incidence
of complications was 35 years.
Figure 1 - Histogram representing age distribution. The average age of the
patients analyzed was 44.6 years. The age with the highest incidence
of complications was 35 years.
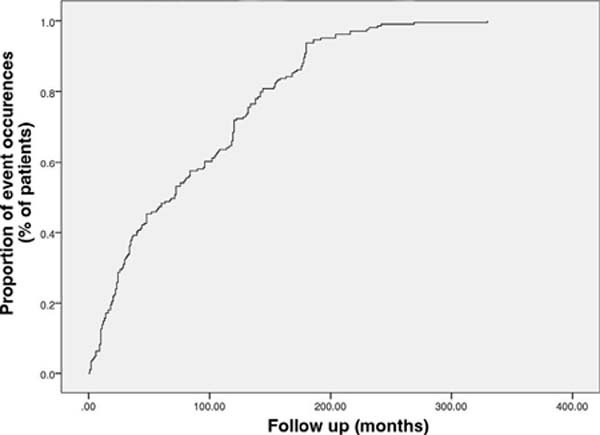
The median time for complications to appear after the initial application of PMMA
to the face was 71 months, with an interquartile range of 23 to 132. The minimum
and maximum times for complications to appear were 1 and 330 months (Figure 2).
Figure 2 - Occurrence of events showing a median time to onset of
complications after the initial PMMA injection of 71 months.
Figure 2 - Occurrence of events showing a median time to onset of
complications after the initial PMMA injection of 71 months.
The malar and mandibular regions were most affected by complications, followed by
the zygomatic region. Approximately half of the patients had complications in
the malar (48.8%) or mandibular (47.8%) region. The zygomatic region appeared as
the third area most affected by complications (43.5%) (Table 1).
Table 1 - Distribution of complications according to area of
occurrence.
| Compromised
area
|
Number of
patients and frequency
|
| Malar |
102 (48.8%) |
| Mandibular |
100 (47.8%) |
| Zygomatic |
91 (43.5%) |
| Chin |
58 (27.8%) |
| Lip |
57 (27.3%) |
| Nasolabial fold |
46 (22%) |
| Nose |
21 (10%) |
| Eyelid |
18 (8.6%) |
| Glabella |
18 (8.6%) |
| Temporal |
15 (7.2%) |
| Front |
4 (1.9%) |
| Ear |
2 (1%) |
Table 1 - Distribution of complications according to area of
occurrence.
In the studied population, 501 complications were found distributed across
various areas of the face. The most frequent complication was granuloma,
followed by edema and inflammation (Table 2). If we only take into account the type of complication, granuloma
was the most common, observed in 135 (64.4%) patients in the population
evaluated. On the other hand, considering the total or absolute number of 501
complications, granulomas represented 26.9%. Complications compromised one or
more anatomical regions of the face. They were present in isolation or
associated with other complications (Table 3). Most patients had only one type of complication, a fact observed
in 84 (40.1%) patients.
Table 2 - Type and frequency of complications.
| Type of
complication
|
Number of
patients and frequency
|
| Granuloma |
135 (64.6%) |
| Edema |
120 (57.4%) |
| Inflammation |
78 (37.3%) |
| Function |
42 (20.1%) |
| Nodule |
40 (19.1%) |
| Neovascularization |
40 (19.1%) |
| Pigmentation |
12 (5.7%) |
| Tearing |
12 (5.7%) |
| Infection |
12 (5.7%) |
| Necrosis |
6 (2.9%) |
| Fistula |
4 (1.9%) |
Table 2 - Type and frequency of complications.
Table 3 - Number of complications per patient.
| No. of
patients
|
Number of
complications
|
| 84
(40.1%)
|
1 |
| 51 (24.1%) |
2 |
| 31
(14.8%)
|
3 |
| 21 (10.0%) |
4 |
| 10
(4.7%)
|
5 |
| 5 (2.3%) |
6 |
| 7
(3.3%)
|
7 or more |
Table 3 - Number of complications per patient.
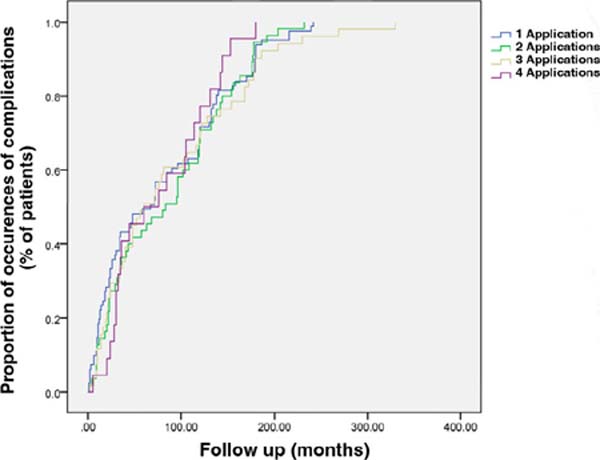
To evaluate the relationship between the number of PMMA injections in the face
and the appearance of complications, the log-rank test was used to compare the
groups. The number of applications ranged from 1 to 5. The p-value of the
log-rank test (p = 0.73) was not statistically significant,
showing no relationship between the variables’ number of PMMA applications and
the emergence of complications (Figure 3).
Figure 3 - Kaplan-Meier curve representing the occurrence of complication
events by the number of applications. The log-rank test did not show
a relationship between the number of times the product was injected
and the time until complications occurred (p=0.73).
Figure 3 - Kaplan-Meier curve representing the occurrence of complication
events by the number of applications. The log-rank test did not show
a relationship between the number of times the product was injected
and the time until complications occurred (p=0.73).
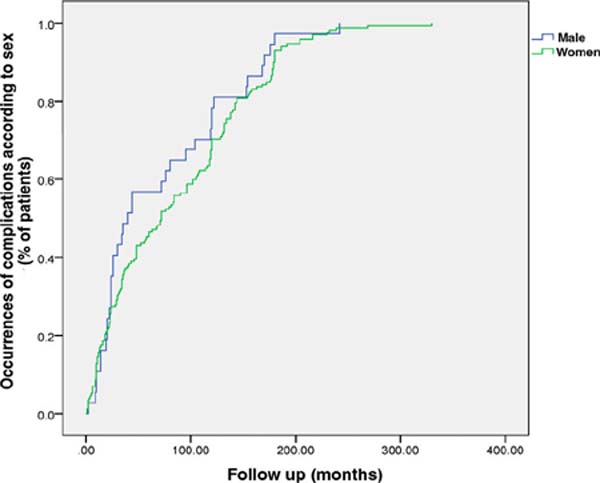
Of the population evaluated, 82.3% were women. No difference was found between
men and women in the time to occurrence of complications (p =
0.27) (Figure 4).
Figure 4 - Time of occurrence of complications according to sex. There was
no difference in the emergence of complications when comparing the
sexes (p=0.27).
Figure 4 - Time of occurrence of complications according to sex. There was
no difference in the emergence of complications when comparing the
sexes (p=0.27).
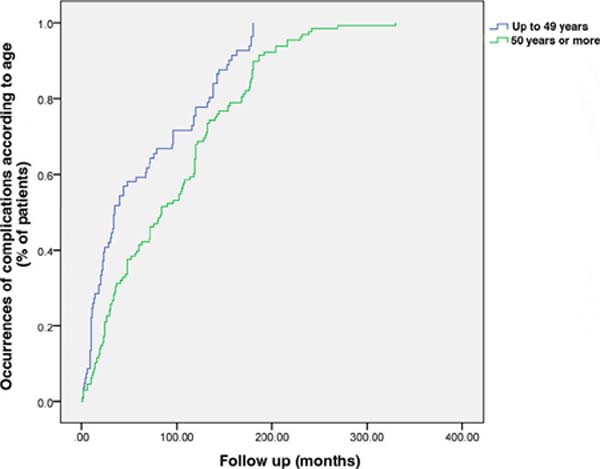
Patients under 50 years of age had earlier manifestations of complications than
those over 50 years of age (p<0.001) (Figure 5).
Figure 5 - Time of occurrence of complications according to age. The group
under 50 years of age had earlier complications than the group over
50 years of age (p<0.001).
Figure 5 - Time of occurrence of complications according to age. The group
under 50 years of age had earlier complications than the group over
50 years of age (p<0.001).
The treatment carried out by more than half of the population evaluated (111
patients, 53.1%) was the injection of corticosteroids in the area compromised by
the complication. The second most used resource in an attempt to treat
complications was surgical removal, performed in 40 (19.1%) of the patients
(Table 4).
Table 4 - Most common treatments used to manage complications.
| Type of
treatment
|
Number of
patients and frequency
|
| Corticosteroid injetable |
111 (53.1%) |
| Surgery |
40 (19.1%) |
| 5-fluorouracil |
23 (11%) |
| Ozone |
23 (11%) |
| Aspiration |
14 (6.7%) |
| Intraoral bichectomy |
13 (6.2%) |
| Xylitol |
8 (3.8%) |
| Allopurinol |
3 (1.4%) |
| Laser |
2 (1%) |
Table 4 - Most common treatments used to manage complications.
DISCUSSION
Applying PMMA as PP to the face may present unwanted results and complications.
The population assessed already had previous injections of PMMA as a predictor
variable and already established complications related to this material in at
least one area of the face.
The diagnosis of complications related to PMMA injection into the face must
follow established evaluation and diagnostic criteria. The differentiation
between nodules and granuloma formation due to a foreign body is evidenced
mainly in the anatomopathological evaluation. From a clinical point of view,
this differential diagnosis between nodules and granulomas is fundamental, as it
allows for a more specific and appropriate therapeutic approach to each
case.
Generally, the nodules tend to appear soon after the application of PMMA and may
disappear after the edema subsides. They appear as single or solitary nodules,
usually on the lips, measuring a few millimeters. Normally, the nodules are
related to a technical application error such as an injection that is too
superficial or too much product. On the other hand, granulomas usually appear
suddenly, generally between 6 and 24 months after their injection. Granulomatous
lesions are evident at the same time in all areas where this product was applied
and not just in one region4, 5.
Our study highlights the evaluation of individuals with complications that arose
many years after the initial injection, 14 of these 20 years or more, and its
importance in identifying the primary and secondary outcomes. Another relevant
factor concerning the follow-up period is the fact that these patients underwent
their initial treatment with different doctors.
Therefore, unlike most publications, which show the personal experience of a
single author or even a small group of doctors in the application and management
of complications with PMMA, the patients included in this study portrayed
complications arising from several professionals. Most likely, this universe of
doctors has a different degree of experience in relation to the application
method, safety, anatomical knowledge, and possible complications at an
undetermined time with this material.
The median time of 71 months with an interquartile range of 23 to 132 months
found in the study is longer than practically all studies published to date.
Many appeared early, others quite late, highlighting the lack of predictability
when the complication occurred. The incidence of granulomas was present in most
patients. This late complication coincides with reports in the literature after
PMMA injection2, 6.
Granulomas were the most frequent complications, diagnosed in 135 (64.6%)
patients. Edema, inflammation, functional changes, nodule formation, and
neovascularization represented other frequent types of complications related to
the use of PMMA on the face. The three most affected regions were the malar,
mandibular, and zygomatic regions. This distribution of complications according
to the area of occurrence on the face coincided with most reports in the
literature7. Although it was not
evaluated in the study, the amount of PMMA injected into each patient or each
anatomical region separately could be related to the greater occurrence of
complications in these three areas8.
Factors related to the late appearance of granulomas, such as the presence of
biofilm, quality, and characteristics of the injected material, and systemic,
autoimmune or hormonal manifestations were not evaluated.
The study showed an average time for complications to appear of 71 months,
practically 6 years after the initial injection of PMMA into the face. The
latest complication occurred after 330 months. Most publications in the medical
literature show the emergence of complications at an earlier period6, 9.
The number of times PMMA was injected was not related to the appearance of the
complications described. The fact that an individual has been subjected to more
than one injection of the product at different times does not necessarily mean
that a greater quantity of it has been used, another factor that could be
associated with complications. The design of this retrospective study, however,
did not include data on injected volume in the studied population.
Women represented the majority of individuals studied (172 cases, 82.3%). When
evaluating the moment of occurrence between the sexes, however, there was no
significant difference in the time of occurrence when compared to men. The
population under 50 years of age presented complications earlier than those over
50 years of age. Despite the hypothesis that this group of younger individuals
may have a more active immune system and reaction, factors related to this
statistical difference that would justify this finding were not evaluated.
The study demonstrated that the use of injectable corticosteroids is the most
frequent treatment. This finding coincides with most of the literature10, 11. Although local injection of corticosteroids presents risks such
as atrophy of subcutaneous cellular tissue, changes in skin pigmentation, and
neoformation of vessels, the apparent simplicity of this resource, its low cost,
and, sometimes, rapid action, probably justify the option for it, making -the
most frequent in the attempt to control complications. The approach to
granulomas represents a late complication that is difficult to manage.
Surgical removal usually reduces the inflammatory aspect and other symptoms
related to the presence of biofilm2, 12. In our study, surgery represented the
second most used resource in the treatment of complications. Although often
resolving, this approach is not free from risks and complications due to the
rich and complex anatomy of the face and the possible changes produced by the
material such as fibrosis, irregularities, or granulomas related to PMMA.
However, this aspect is little reported in the literature.
The use of laser in the treatment of PMMA-related complications was used in our
case series study in only 2 patients. Factors such as still scarce and recent
publications, lack of knowledge about laser technology and its application in
medicine still limited to a few medical specialties, availability generally
restricted to large urban centers, combined with the high cost of equipment, are
possibly related to this small number of patients undergoing the laser
treatment13, 14, 15, 16, 17.
Although not included in this study, variables such as smoking, testing of
inflammatory biomarkers and antibodies showing immunological impairment or
interaction for certain complications, or even post-COVID reactions could
clarify other factors potentially related to unwanted effects with PMMA18, 19. Furthermore, the presence of biofilm, clinical, inflammatory, or
infectious complications, the quality of the material, or the expertise of the
injecting physician could also be related to complications.
CONCLUSION
The study showed important data regarding incidence, time of onset, compromised
anatomical region, and management of complications, among other relevant
aspects. Despite representing only a small portion of the population affected by
problems related to PMMA injection into the face, the results serve as a
knowledge base for a better understanding of these problems that are difficult
to predict and treat. More studies are necessary to better clarify the
complications and factors related to them.
REFERENCES
1. Urdiales-Gálvez F De cabo-Francés FM, Bové I. Ultrasound patterns of
different dermal filler materials used in aesthetics. J Cosmet Dermatol.
2021;20(5):1541-8.
2. Urdiales-Gálvez F Delgado NE, Figueiredo V, Lajo-Plaza JV, Mira M,
Moreno A, et al. Treatment of Soft Tissue Filler Complications: Expert Consensus
Recommendations. Aesthetic Plast Surg. 2018;42(2):498-510.
3. Trinh LN, McGuigan KC, Gupta A. Delayed Complications following
Dermal Filler for Tear Trough Augmentation: A Systematic Review. Facial Plast
Surg. 2022;38(3):250-9.
4. Mundada P, Kohler R, Boudabbous S, Toutous Trellu L, Platon A,
Becker M. Injectable facial fillers: imaging features, complications, and
diagnostic pitfalls at MRI and PET CT. Insights Imaging.
2017;8(6):557-72.
5. Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M,
Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal
fillers: part 1. Possible causes. Plast Reconstr Surg.
2009;123(6):1842-63.
6. Paulucci BP. PMMA Safety for Facial Filling: Review of Rates of
Granuloma Occurrence and Treatment Methods. Aesthetic Plast Surg.
2020;44(1):148-59.
7. Machado RA, Oliveira LQ Martelli-Júnior H, Pires FR, Carvas JB,
Rogerio VE, et al. Adverse reactions to the injection of face and neck aesthetic
filling materials: a systematic review. Med Oral Patol Oral Cir Bucal.
2023;28(3):e278-84.
8. Blanco Souza TA, Colomé LM, Bender EA, Lemperle G. Brazilian
Consensus Recommendation on the Use of Polymethylmethacrylate Filler in Facial
and Corporal Aesthetics. Aesthetic Plast Surg.
2018;42(5):1244-51.
9. Souza RN, Mendoça SG, Alencar EC, França ALA, Araújo ÊG, Leite LAS.
Late complication of cutaneous filling after a facelift: a case report. Rev Bras
Cir Plást. 2016;31(2):269-72.
10. Singh K, Nooreyezdan S. Nonvascular Complications of Injectable
Fillers-Prevention and Management. Indian J Plast Surg.
2020;53(3):335-43.
11. Pompeu M, Lima V, Magalhaes HL, Regattieri N, Costa IMC. Foreign
body granuloma treatment with 22-MHz ultrasound-guided corticoid infiltration. J
Cosmet Dermatol. 2019;18(3):908-9.
12. Durkin AJ, Catena D, Woltjen N, Boyle K, Polling M, Weng J, et al.
Surgical Management of Polymethylmethacrylate-Collagen Gel Complications in the
Lower Eyelid: A Case Series. Ann Plast Surg. 2023;90(1):12-8.
13. Goldman A, Wollina U. Intralesional Neodymium YAG laser to Treat
Complications of Polymethylmethacrylate. Open Access Maced J Med Sci.
2018;6(9):1636-41.
14. Goldman A, Wollina U. Polymethylmethacrylate-induced nodules of the
lips: Clinical presentation and management by intralesional neodymium: YAG laser
therapy. Dermatol Ther. 2019;32(1):e12755.
15. Goldman A, Wollina U, Machado D, Marionwic D. Laser in the Treatment
of Granulomas on the Nose Produced by Polymethylmethacrylate: A Case Series. J
Drugs Dermatol. 2021;20(11):1161-6.
16. Piccolo D, Mutlag MH, Pieri L, Pennati BM, Conforti C, Bonan P.
Novel Management of Granuloma Formation Secondary to Dermal Filler with
Intralesional 1444 nm Nd:YAG Laser Technique. Medicina (Kaunas).
2023;59(8):1406.
17. Schelke LW, Decates TS, van der Lugt CIM, Pelzer L, de Mey G,
Velthuis PJ. Intralesional Laser Treatment for Dermal Filler Complications.
Plast Reconstr Surg. 2018;141(6):1361-9.
18. Freire de Carvalho J. Polyautoimmunity (Psoriasis, Sjogren’s
syndrome, and autoimmune uveitis) following polymethylmethacrylate injection.
Eur Rev Med Pharmacol Sci. 2021;25(6):2478-80.
19. Vengalil N, Council LM, Michalski BM. Foreign body granulomas to
polymethylmethacrylate soft tissue filler following COVID-19 infection. JAAD
Case Rep. 2023;41:1-3.
1. Hospital São Lucas da PUCRS, Porto Alegre, RS, Brazil
2. Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil
3. Universidade do Vale do Rio dos Sinos, São Leopoldo, RS, Brazil
Corresponding author: Alberto Goldman
Av. Augusto Meyer 163, conj. 1203, Porto Alegre, RS, Brazil. Zip Code: 90.550-110,
E-mail: alberto@goldman.com.br
Article received: November 11, 2023.
Article accepted: April 30, 2024.
Conflicts of interest: none.
Institution: Hospital São Lucas da PUCRS; Clínica Goldman Cirurgia Plástica, Porto Alegre, RS, Brazil.