INTRODUCTION
Scars are generally a matter of concern for patients undergoing surgical
procedures, especially if they are likely to appear in more visible areas of
the
body1. Surgical wounds
in regions such as the face tend to have a greater aesthetic impact during the
healing process, and there may also be greater tension in the surgical wound
in
some myofascial structures, resulting in scars2.
Keloid is characterized as a scar of considerable thickness, raised, resulting
from the abnormal growth of scar tissue, which, unlike hypertrophic scars,
extends beyond the limits of the surgical wound3. Due to their physiology, keloids do not develop
in animals, which makes the process of developing new therapies difficult, as
testing on animals cannot be carried out1. Furthermore, the presence of scars can lead to
biopsychosocial repercussions, whether physiological or social limitations due
to aesthetics4. With these
aspects in mind, several treatments are used to reduce these problems.
There is still no consensus on a single treatment that is considered the best
alternative for keloid scars. The Virtual Health Library5, with support from the
Sociedade Brasileira de Cirurgia Dermatológica and the
Sociedade Brasileira de Dermatologia, presents the main
treatments for keloids: local radiotherapy, silicone plates, drug injections,
occlusive tapes, surgery, cryotherapy, and laser therapy. These treatment
options mainly aim to reduce symptoms, with their regression or reduction being
less frequent alternatives that are still being studied.
Aiming for better therapy, research using botulinum toxin is gaining more and
more space. Botulinum toxin type A (BTA) acts to reduce tension at the edges
of
surgical wounds during the healing process, thus contributing to improving the
scar aspect, and reducing the possibilities of development and/or progression
of
keloids6.
OBJECTIVE
With this in mind, the present study aims to present a scoping review on the
therapeutic use of botulinum toxin for the treatment of keloid scars.
METHOD
The PICO model, based on Santos et al.7, was used to formulate the guiding questions of this
study, considering: (P) studies that considered patients with keloid scars, (I)
studies in which the main objective was to perform or describe interventions
and
strategies using botulinum toxin for these patients, (C) studies with or without
a control group, (O) studies that reported the development and results of
interventions in the short, medium and long term. Studies carried out until
September 2021 were included in this review if they met the PICO criteria.
The review was constructed following the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses extension for Scoping Reviews: PRISMA-ScR8. The search was carried out in
the PubMed/Medline, Virtual Health Library (VHL), and SciELO databases to
identify articles on the treatment of keloid scars with botulinum toxin. The
search was carried out by combining the terms “botulinum toxin”, “keloid”,
“scar” and “treatment”. The terms were used in combination, according to the
order mentioned above. The terms are based on descriptors present in the Health
Sciences Descriptors (DECs).
Articles of any design, except reviews, in any language were considered, as long
as they were related to the central theme. The exclusion criteria were:
unpublished reports, literature reviews, symptom assessment studies, articles
published in the period before 2016, and studies with no full text. Articles
that met the eligibility criteria were selected based on title and abstract by
two reviewers and articles that did not meet the inclusion criteria were
excluded. After title and abstract screening, studies were submitted to a public
reference manager (Mendeley v.1.17.9) to eliminate duplicates. The result of
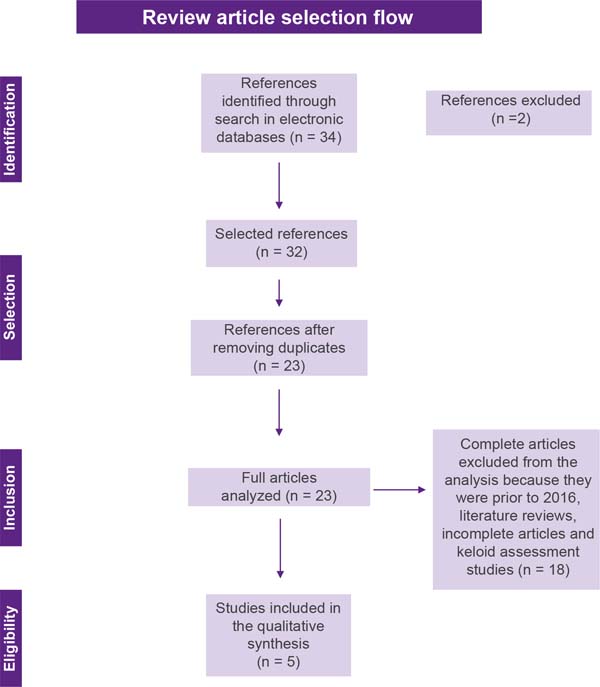
this selection can be seen in Figure 1.
Figure 1 - Flowchart for selecting review articles.
Figure 1 - Flowchart for selecting review articles.
Subsequently, the remaining full-text articles were examined by a third reviewer.
Any disagreement was resolved through discussion until consensus was reached,
or
with the involvement of a fourth reviewer. Then, the following points were
extracted from each study, when available: authorship, year of publication,
title, objectives, and results. These data were arranged in tables in Microsoft
Word 2016, for final inclusion analysis.
RESULTS
The initial literature search found 34 studies. Of these, 12 studies were
identified using PubMed/Medline, 20 using the VHL, and 2 in SciELO. After
selection by title and abstract, 32 articles were run in Mendeley to eliminate
duplicates. The resulting 23 full-text articles were reviewed to establish
whether the publication met the inclusion criteria and 5 were considered
eligible (Figure 1).
Of the 5 articles eligible for this review, 1 is a cohort study, 2 are case
reports, 1 is a randomized clinical trial and 1 is a case-control study (Chart 1). The search strategy and study
inclusion and exclusion criteria are detailed in Figure 1.
Chart 1 - Presentation of studies according to year, authorship, and type of
study.
| Reference |
Title |
Study type |
| Cardoso et al.
(2016)2 |
Application of
botulinum toxin in secondary intention healing
|
Case report |
| Pruksapong et al. (2017)9 |
Efficacy of Botulinum Toxin A in Preventing
Recurrence of Keloids: Double Blinded Randomized Controlled
Trial Study: Intraindividual Subject
|
Randomized Clinical Trial |
| Zhou et al.
(2017)10 |
Evaluation on
efficacy and adverse reactions of combined therapy with
botulinum toxin type A in treatment of keloid
|
Case-control |
| Rasaii et al. (2019)11 |
Intralesional triamcinolone alone or in
combination with botulinium toxin A is ineffective for the
treatment of formed keloid scar: A double blind controlled pilot
study
|
Cohort |
| Pires et al.
(2020)12 |
Botulinum toxin
type A in the treatment of hypertrophic burn scars in pediatric
age:
Clinical Case
|
Case report |
Chart 1 - Presentation of studies according to year, authorship, and type of
study.
Regarding treatments, the studies present variations concerning their
populations, methods, and clinical criteria, as shown in Chart 2.
Chart 2 - Presentation of studies according to authorship and methods.
| Reference |
Method |
Clinical
criteria
|
| Cardoso et al.
(2016)2 |
N=1
Age
= 36 years Botulinum toxin
application type A
|
Scar resulting
from Mohs micrographic surgery in the supralabial region;
Operative scar measuring 3 x 1.6cm;
Application in the
immediate postoperative period
|
| Pruksapong et al. (2017)9 |
N=25 patients, 50 keloids
Average age = 26
years
Control group = injection of corticosteroid
therapy
Study group = toxin botulinum type A
|
Present two scars keloids;
Not being
pregnant or breastfeeding;
Scars larger than 10cm; not be
allergic to toxin, lidocaine;
Do not present undesirable
medical conditions and use anticoagulant drugs or antiplatelet
agents
|
| Zhou et al.
(2017)10 |
N=58
Control group = injection of betamethasone and
topical hyaluronic acid
Study group = toxin combined type
A botulinum with injection of betamethasone and topical
hyaluronic acid
|
Present scars
keloids;
No restrictions on the pharmacological
components of the study
|
| Rasaii et al. (2019)11 |
N=23
Average age = 23 years
Control group = intralesional triamcinolone acetonide
plus placebo
Study Group = botulinum toxin type A
combined with saline solution
|
Present two scars keloids;
Do not be
pregnant
or breastfeeding;
Absence of
neuromuscular junction disease or use of neuromuscular junction
blockers
|
| Pires et al.
(2020)12 |
N=1
Age
= 12 years
Application of botulinum toxin type A with
prior topical analgesia with lidocaine + prilocaine 25 mg/g
cream
|
Scars resulting
from 2nd and 3rd burns on the face, scalp,
ear pinna, neck, anterior side of the chest, and upper limbs;
Application to the right axillary scar and radial border
of the first finger of the
right hand;
Application 5 months post-burn
|
Chart 2 - Presentation of studies according to authorship and methods.
The selected studies present different outcomes and conclusions regarding the use
of BTA. In Chart 3 it is possible to
observe the treatments used, results, and conclusions of the studies
analyzed.
Chart 3 - Studies according to authorship, treatment, results, and
conclusions.
| Reference |
Treatment |
Results |
Conclusions |
| Cardoso et al.
(2016)2 |
8 units of BTA
in the surgical wound, with healing by secondary intention.
|
Complete wound
healing in 18 days with formation of slightly erythematous scar
tissue on the upper lip, with slight extension to the
supralabial region, maintained in the late postoperative period,
favoring aesthetics and functionality.
|
The molecular
properties of BTA suggest that its action is best at the
beginning of healing, when the fibroblasts are still in the
proliferative phase and have intense apoptotic activity,
requiring further studies on this process in secondary
healing.
|
| Pruksapong et al. (2017)9 |
Control group = Injection of triamcinolone
acetonide (10mg/cc) seven days after stitch removal, repeated in
the first, second, and third months.
Study group =
intradermal BTA, with a dose of 1.5 units / 1cm length (Botox®
50 units of toxin with 0.9% NSS for injection 2.5cc,
concentration 2 units per 0.1cc) seven days after stitch removal
(one dose).
|
In the first and third months, the outcome in the
toxin group was more favorable than in the control group, while
the outcome in the control group was more favorable than in the
toxin group in the sixth month of follow-up.
|
The use of BTA is significantly better at
preventing keloids from recurring when compared to
corticosteroid therapy after one and three months. However,
corticosteroid therapy offers significantly better results at
6-month follow-up.
|
| Zhou et al.
(2017)10 |
Control group =
Betamethasone and topical hyaluronic acid
injection.
Study group = botulinum toxin type A combined
with betamethasone and topical hyaluronic acid
injection.
Both groups of patients were locally injected
with betamethasone once every 4 weeks, 3 consecutive times, and
topical hyaluronic acid was used daily. Patients in the combined
treatment group were injected with botulinum toxin type A into
the periphery of the skin lesion after the first injection of
betamethasone.
|
The aesthetics
of the skin lesions in the study group improved better after 3
applications. Pain and itch scores in the control group
decreased during 1 month of treatment but gradually increased at
2 and 3 months; in contrast, in patients in the study gradually
decreased within 3 months of treatment, this difference being
statistically significant.
Over the course of 3 months of
treatment, the thickness of skin lesions in the study group
gradually decreased, but the reduction in the control group was
not significant. The pain, itching symptoms, and skin thickness
of patients in the control group increased 2 weeks after each
injection, while the combination treatment group did not
experience recurrence. The incidence of adverse reactions in the
control
group was 26.7% and in the study group it was
25.0%, with no
significant difference between
the two groups.
|
BTA combined
with local injection of betamethasone and topical hyaluronic
acid for the treatment of keloids is more effective than local
injection of betamethasone and topical hyaluronic acid alone,
and there is no significant difference in the incidence of
adverse reactions, being favorable for clinical
application.
|
Chart 3 - Studies according to authorship, treatment, results, and
conclusions.
DISCUSSION
Aesthetic and functional issues related to scars, especially keloids, end up
generating discomfort and dissatisfaction on the part of people who have them.
Studies such as that by Motoki et al.13 present negative results from interviewees concerning
dimorphic disorders of the self-concept and the body, and also state that people
with keloids in socially more seen regions such as the face, chest, and upper
limbs report a greater negative impact on their body image.
The development of treatment strategies for this condition is a challenge for the
scientific community since keloids do not develop in animals, which limits the
possibilities for research and testing of new therapeutic elements1. As possibilities, the clinical
and scientific community uses strategies already tested in other conditions,
in
addition to studies with in vitro cells to generate new options14. Lee et al.15 present combined therapy as
the main alternative in the treatment of keloids, whether this therapy involves
lasers, cryotherapy, or intralesional drug injection, presenting greater safety
and efficacy when compared to individual monotherapies.
As an emerging therapeutic, the use of botulinum toxin type A is gaining
increasing attention from the clinical and scientific communities. This greater
interest can be observed when this work initially found 34 studies that related
the use of BTA for the treatment of keloids. In this review, five studies were
eligible, ranging from cohort studies, case reports, randomized clinical trials,
and case-control studies. Due to the previously mentioned difficulties in
developing new research in the area, there is still little variation in the
types of studies, which can be seen as an obstacle to treating the
condition.
The studies analyzed present variations in their populations and clinical
criteria, but it is possible to observe that in two studies10,11 combined therapy was used, as advocated by Lee et
al.15, these two
studies presented results favorable to the replication of combined therapy for
treatment of keloid. Concerning samples and methodologies, the absence of
application protocols and considerable methodological deficiencies can be
highlighted, mainly regarding sample size and uniformity of treatments.
It was also observed that the studies highlighted the exclusion of participants
who are allergic to the components of the treatment, pregnant and lactating
women, who use anticoagulant or antiplatelet drugs, as well as those with
neuromuscular junction disease or the use of neuromuscular junction blockers,
being the effectiveness of treatment with BTA has not been tested in these
populations, therefore, without indication of scientific evidence and clinical
replicability for them9-11.
The five studies applied BTA intradermally, either at the edge of the
scar/operative wound or directly at the site. Sohrabi & Goutos16 add to these studies when they
state, in their review, that other research also points to the application of
BTA in keloids as a growing treatment to minimize tension on the scar edge and
optimize the activity of fibroblasts, directly implicated in the pathogenesis
of
the formation of scars. In the present study, variation in the dosage of BTA
was
still observed. As it is composed of studies with different populations, ages,
and clinical conditions, this review was unable to define a dosage standard for
the toxin, as this dosage is linked to and dependent on the clinical
manifestation, size of the scar, and the event that triggered the healing
process.
Despite these variations, the five studies observed are related to the findings
resulting from BTA-based therapy. It is possible to highlight as the main
results the acceleration of the healing process of surgical wounds and reduction
of scar formation2, significant
short-term results in the reduction of keloids9, and improvement and maintenance of aesthetic,
functional, and symptomatic aspects, especially pain and itching10-12. In other reviews5,16 it was also
possible to find the replicability of these results, with the use of BTA for
keloid a possibility being of effective clinical treatment and with evidence
already presented in clinical and scientific circles.
The use of botulinum toxin type A for the treatment of keloid scars is justified
mainly by its chemoimmobilizing mechanisms of the muscles in the region, and
its
action on fibroblastic activity. Studies conclude that the use of botulinum
toxin type A has a better effect at the beginning of the healing process, with
direct action on fibroblasts2,
that this treatment presents fewer adverse reactions and better short-term
results when compared to other injectable pharmacological treatments9,10, that this alternative acts better in managing symptoms
in different populations and clinical manifestations5, even in pediatric age16, thus encouraging the clinical community to
consider BTA as a therapeutic alternative for selected and well-analyzed cases
of keloids, always taking into account the clinical particularities and
manifestation of the condition.
CONCLUSION
The most recent studies suggest a good potential for the use of botulinum toxin
type A for the treatment of keloid scars, mainly for short-term results, and
reduction of local symptoms such as pain and itching when compared to other
pharmacological treatments. However, there are deficiencies in the studies as
they have small populations, short follow-up periods, and lack of homogeneity
in
the results found. Therefore, it is necessary to develop larger studies with
better methodologies, aiming to better define the use of BTA for the treatment
of keloids in different situations, and the development of unified scar
management protocols for better clinical replicability.
REFERENCES
1. Ogawa R. Keloid and Hypertrophic Scars Are the Result of Chronic
Inflammation in the Reticular Dermis. Int J Mol Sci.
2017;18(3):606.
2. Cardoso AS, Teixeira DA, Oliveira BV, Carneiro PP, Junqueira RF.
Botulinum toxin application in the secondary intention healing. Surg Cosmet
Dermatol 2016;8(2):163-6.
3. Hochman B, Farkas CB, Isoldi FC, Ferrara SF, Furtado F, Ferreira LM.
Distribuição de queloide e cicatriz hipertrófica segundo fototipos de pele de
Fitzpatrick. Rev Bras Cir Plást. 2012;27(2):185-9.
4. Gouveia BN, Ferreira LD, Rocha Sobrinho HM. O uso da toxina
botulínica em procedimentos estéticos. Rev Bras Mil Ciênc.
2020;6(16):56-63.
5. Brasil. Ministério da Saúde. Biblioteca Virtual em Saúde. Queloide.
2021. Elaborado por: Sociedade Brasileira de Cirurgia Dermatológica e Sociedade
Brasileira de Dermatologia. Brasília: Ministério da Saúde; 2021 [acesso 2021
Out
30]. Disponível em: https://bvsms.saude.gov.br/queloide/
6. Kasyanju Carrero LM, Ma WW, Liu HF, Yin XF, Zhou BR. Botulinum toxin
type A for the treatment and prevention of hypertrophic scars and keloids:
Updated review. J Cosmet Dermatol. 2019;18(1):10-5.
7. Santos CMC, Pimenta CAM, Nobre MRC. The PICO strategy for the
research question construction and evidence search. Rev Latino Am Enferm.
2007;15(3):508-11.
8. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et
al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and
Explanation. Ann Intern Med. 2018;169(7):467-73.
9. Pruksapong C, Yingtaweesittikul S, Burusapat C. Efficacy of
Botulinum Toxin A in Preventing Recurrence Keloids: Double Blinded Randomized
Controlled Trial Study: Intraindividual Subject. J Med Assoc Thai.
2017;100(3):280-6.
10. Zhou M, Wang L, Jiang R, Zhu M, Chen F. Evaluation on efficacy and
adverse reactions of combined therapy with botulinum toxin type A in treatment
of keloid. J Jilin Un (Medicine Ed.). 2017;6:386-90.
11. Rasaii S, Sohrabian N, Gianfaldoni S, Hadibarhaghtalab M, Pazyar N,
Bakhshaeekia A, et al. Intralesional triamcinolone alone or in combination with
botulinium toxin A is ineffective for the treatment of formed keloid scar: A
double blind controlled pilot study. Dermatol Ther.
2019;32(2):e12781.
12. Pires M, Soudo A, Costa MJ. Toxina Botulínica Tipo A no Tratamento
das Cicatrizes Hipertróficas por Queimadura em Idade Pediátrica: Caso Clínico.
Rev Soc Port Med Fís Reab. 2020;32(3):126-9.
13. Motoki THC, Isoldi FC, Brito MJA, Filho AG, Ferreira LM. Keloid
negatively affects body image. Burns. 2019;45(3):610-4.
14. Dai X, Lei TC. Botulinum toxin A promotes the transdifferentiation
of primary keloid myofibroblasts into adipocyte-like cells. Basic Clin Pharmacol
Toxicol. 2021;129(6):462-9.
15. Lee YI, Kim J, Yang CE, Hong JW, Lee WJ, Lee JH. Combined
Therapeutic Strategies for Keloid Treatment. Dermatol Surg.
2019;45(6):802-10.
16. Sohrabi C, Goutos I. The use of botulinum toxin in keloid scar
management: a literature review. Scars Burn Heal.
2020;6:2059513120926628.
1. Universidade Estadual do Ceará, Fortaleza,
Ceará, Brazil
2. Santa Casa de Santos, Santos, São Paulo,
Brazil
Corresponding author: Eduardo Lafayette Monteiro
Av. Senador Ruy Carneiro, 212, Miramar, João Pessoa, PB, Brazil, Zip Code:
58032-101, E-mail: eduardomlafayette@gmail.com