INTRODUCTION
Patients undergoing breast surgery are at a greater risk of being unable to breastfeed.
Milk production is not expected when all breast tissues are removed – as is the case
with mastectomies. This brief report describes a rare case of a woman who could breastfeed
following radical mastectomy, latissimus dorsi flap, and implant reconstruction.
Milk production and secretion depend on the mammary gland tissue being present and
functioning properly.1 Patients undergoing radical mastectomy have the milk-producing tissue removed, rendering
breastfeeding impossible.2
We present a case of a breast cancer patient who underwent late breast reconstruction
with a latissimus dorsi flap (LDF) and implant following modified radical mastectomy
(MRM). The patient subsequently became pregnant and presented milky secretion via
the reconstructed nipple-areola complex (NAC). The COVID-19 pandemic isolation precluded
laboratory analysis of the secretion, and it was impossible to confirm that it was
milk.
CASE REPORT
A 28-year-old woman, nulliparous, non-smoker, and free of comorbidities, came to us
for late right breast reconstruction. At 25, she was diagnosed with invasive ductal
carcinoma in the right breast, Grade 3, cT3N2aM0, CS-IIIA (Immunohistochemistry: Ki67+;
HER2 score 3+; RE-; RP-).
She received four cycles of neoadjuvant chemotherapy with adriamycin and cyclophosphamide
over two months. Four additional cycles of paclitaxel and trastuzumab consolidation
were performed. Following chemotherapy, she underwent MRM, axillary lymphadenectomy,
and immediate breast reconstruction with a tissue expander. The anatomopathological
examination revealed grade II invasive carcinoma with clear margins, no angiolymphatic
invasion, carcinoma foci in situ, and no papillary infiltration. Five lymph nodes
were examined, and all were found to be normal. There was no mention of tumor size;
only final staging was mentioned (ypT1bN0M0).
Soon after the expansion was completed, the patient received maintenance chemotherapy
with trastuzumab (completing 18 doses) and adjuvant radiotherapy. She received external
radiotherapy with a 6MV linear accelerator using a 3D technique. The adjuvant dose
prescribed was 50.4 Gy in the right breast and supraclavicular fossa (fractions of
180cGy). The treatment was halted on the final day due to radiodermatitis after administering
a total dose of 48.6Gy. According to the report, she evolved as her radiodermatitis
worsened; the expander extruded and was, therefore, removed.
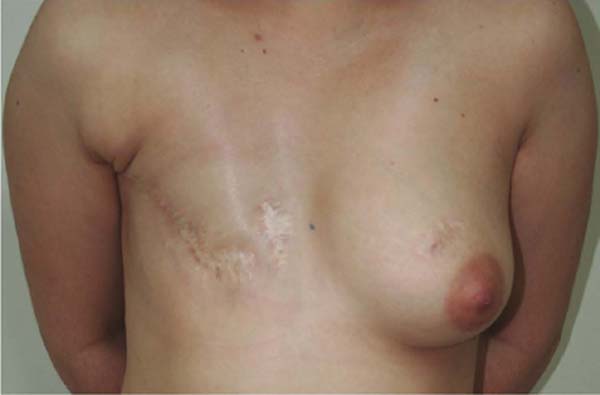
She contacted us 18 months after undergoing a mastectomy for late breast reconstruction
(Figure 1). An LDF procedure was performed by placing a 320ml textured implant (Silimed™, Natural,
extra-high projection).
She was satisfied with the outcome one year after surgery and did not desire breast
symmetrization procedures (Figure 2). Following that, NAC reconstruction was performed. The areola was reconstructed
using a total skin graft from the medial thigh and the papilla was reconstructed using
a graft from the contralateral papilla’s caudal portion. The patient lived in another
city and did not return for follow-up after removing the sutures.
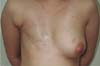
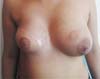
Figure 2 - One-year postoperative after LDF associated with a 320ml extrahigh projection implant.
Figure 2 - One-year postoperative after LDF associated with a 320ml extrahigh projection implant.
She contacted us six months later to inform us that she had become pregnant. Following
the child’s birth in February 2020, she contacted us again to report that she was
breastfeeding normally on the left breast despite the caudal papilla being removed
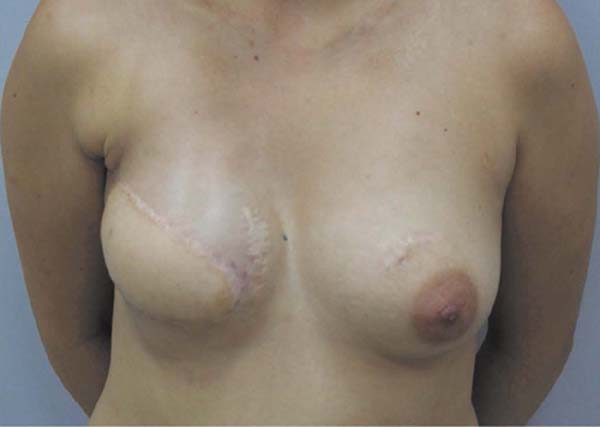
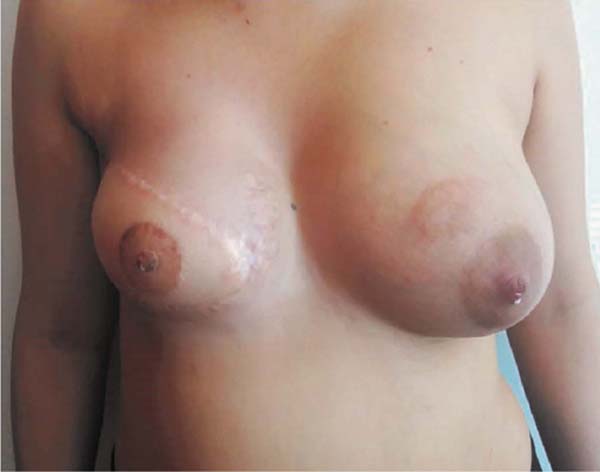
and that the right breast produced milky secretion in small amounts (Figures 3 and 4, Video 1).
Figure 3 - 18 months after NAC reconstruction; one month after delivery. Flow of milk through
both nipples.
Figure 3 - 18 months after NAC reconstruction; one month after delivery. Flow of milk through
both nipples.
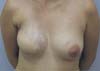
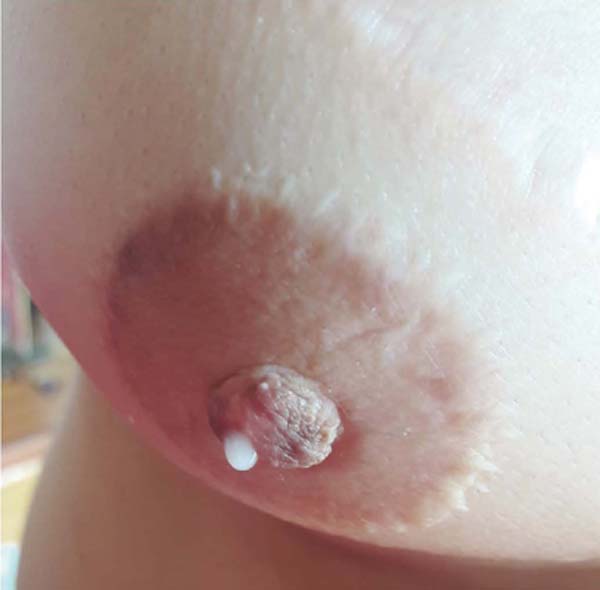
Figure 4 - Right nipple close-up showing milk flow through reconstructed NAC.
Figure 4 - Right nipple close-up showing milk flow through reconstructed NAC.
Video 1 - Milk output through reconstructed NAC.
Video 1 - Milk output through reconstructed NAC.
Due to the imposed social isolation caused by the COVID-19 pandemic, the patient could
not return for imaging tests. The patient reported breastfeeding continuously for
five months until July 2020.
DISCUSSION
Breastfeeding is inversely proportional to the amount of tissue removed following
breast reduction.2,3 When all breast tissue is removed, milk production is not expected.2
Madeira et al.4 described a patient with Poland Syndrome who underwent LDF and implant reconstruction.
Five years later, she became pregnant and successfully breastfed.4 However, that patient may have exhibited rudimentary remnants of breast tissue connected
to the NAC.4 Conversely, our patient underwent a radical mastectomy, radiotherapy, and NAC reconstruction
over the LDF’s cutaneous island.
Sakai and Sakai5 described a nipple-sharing technique that consists of harvesting the outer layer
of the donor nipple and rolling this tissue into a spiral shape before grafting it.
This technique is expected to make breastfeeding viable, as it is supposed to preserve
the donor’s anatomy. In our case, however, the caudal portion of the papilla was utilized,
reducing the likelihood of breastfeeding. Nevertheless, some ducts may have been left
uncut, allowing milk ejection.
The infant’s suction of the NAC stimulates its nerve endings to secrete prolactin
and oxytocin, thereby inducing milk production and ejection.1 One might hypothesize that the contralateral papilla fragment grafted to the reconstructed
breast produced milky secretion in response to hormonal stimuli, as it is the only
structure striving from breast tissue. This, however, contradicts the current understanding
of lactation physiology. Due to the rarity of this situation, surgeons should be open
to alternative theories, including construction morphology, bioelectricity, and the
morphogenetic field.6,7
CONCLUSION
MRM, irradiation, total breast reconstruction using LDF in conjunction with the implant,
and reconstruction of the NAC over the cutaneous island of the LDF all make the production
of a milky secretion unlikely. Additional research is necessary to elucidate this
unusual occurrence.
1. Universidade Federal de São Paulo, São Paulo, S P, Brazil.
2. Universidade do Vale do Sapucaí, Pouso Alegre, MG, Brazil.
Corresponding author: Daniela Francescato Veiga Av. Prefeito Tuany Toledo, 470, Fatima, Pouso Alegre, MG, Brazil Zip code: 37550-000
E-mail: danielafveiga@gmail.com