INTRODUCTION
Breast implants using silicone prostheses began in 1962, and since their implementation,
several studies have been carried out to discover and analyze the impacts of these
substances on the body1. In this respect, there has been a considerable increase in cases associated with
the body’s immune responses, such as the syndrome called ASIA - adjuvant-induced autoimmune/inflammatory
syndrome (or simply “Schoenfeld syndrome”), which manifests itself as an immune reaction
provoked by triggering compounds in genetically predisposed individuals2. Among the etiologies linked to this syndrome, exposure of the body to silicone components
stands out due to its wide use in breast implants.
Silicone is made of polymeric silica, the adjuvant that will activate the immune and
inflammatory system. From the capture of these particles by macrophages, the release
of interleukins 1b (IL-1B) from B cells, Th17 cells, and, subsequently, a clonal expansion
of T3 lymphocytes is induced. Thus, the presence of silicone may lead to the formation
of autoantibodies, polyclonal hypergammaglobulinemia, and progression to lymphoma4.
ASIA presents systemic symptoms such as chronic fatigue, dyspnea, arthralgia, myalgia,
and dysphagia. However, it is important to emphasize that the syndrome also predisposes
patients to develop other autoimmune diseases, especially rheumatic diseases, hypo-
or hyperthyroidism, rheumatoid arthritis, Sjögren’s syndrome, fibromyalgia and systemic
lupus erythematosus3,5.
Another consequence of breast implants is their association with anaplastic large
cell lymphoma (BIA-ALCL)6. The development of BIA-ALCL seems to be associated with three factors: the type
of breast implant, genetic predisposition, and biofilm formation (contamination)2.
Today, two theories explain the pathology of BIA-ALCL: the first is due to the development
of a seroma, with the formation of a periprosthetic effusion around the breast prosthesis
or even inside the implant; the second by the infiltrative unfolding of the disease
itself, with tumor growth inside or outside the capsule7.
The first theory was identified as the most common, according to Groth & Graf7, which will have a late clinical presentation and may manifest from a palpable mass
to lymph node involvement. It is also estimated that ALCL symptoms appear on average
9 years after the implant, enough time to track the disease7.
In addition to the inflammation generated by the compound itself, the patient may
experience “silicone leakage” – usually due to the natural wear and tear of the prosthesis
– caused by the movement of low molecular weight compounds through the envelope of
the implant’s elastomer8.
Given this, the present study aims to analyze the main adverse reactions and symptoms
caused by the immune response of silicone implants concerning ASIA and BIA-ALCL, understanding
how their mechanism of action works.
OBJECTIVE
The article aims to collect data on symptoms, prevalence, and pathophysiology of the
main complications related to silicone implants, such as ASIA and BIA-ALCL (Chart 1).
Chart 1 - Main affections found in the bibliographic review. *ASIA (Adjuvant-Induced Autoimmune
Syndrome) BIA-ALCL (Anaplastic Large Cell Lymphoma Associated with Breast Implants).
| ASIA |
BIA-ALCL |
| Chronic fatigue |
Breast pain |
| Arthralgia |
Palpable mass |
| Myalgia |
Late effusion |
| Dyspnea |
Periprosthetic effusion |
| Dysphagia |
Breast asymmetry |
| Sjogren's syndrome |
Lymph node involvement |
| Fibromyalgia |
|
| Systemic lupus erythematosus |
|
Chart 1 - Main affections found in the bibliographic review. *ASIA (Adjuvant-Induced Autoimmune
Syndrome) BIA-ALCL (Anaplastic Large Cell Lymphoma Associated with Breast Implants).
METHOD
This research addressed the current factors that most contribute to the development
of ASIA and BIA-ALCL in women, both in Brazil and other countries. In order to guarantee
the reproducibility of the analyzed information, 5 selection steps were established,
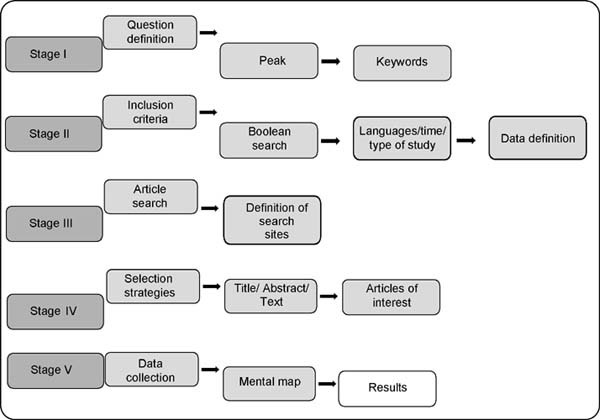
outlined in the flowchart below (Figure 1):
Figure 1 - Methodology employed and its 5 steps.
Figure 1 - Methodology employed and its 5 steps.
Concerning ASIA, Step I was built around the question: “What is the relationship between
the immune response and the symptomatology of the disease?”. Regarding the BIA-ALCL,
for Stage I, the research question in question was defined: “What is the correlation
between anaplastic large cell lymphoma and breast implants?” both questions were obtained
via the PICO method.
For both, Step II consisted of defining the Boolean scheme that met the resolution
of the research problem, as well as definitions of eligibility of articles, such as:
[I] Have a maximum of 11 years of publication; [II] Not present conflicts of interest;
[III] Direct relationship with the object of study and with its guiding question;
[IV] Availability in Portuguese, English, French, and Spanish. It was also established
that the initial Boolean search criteria would be from the terms:
For ASIA: “ASIA breast silicone,” “Breast implant,” “silicone disease,” and “Autoimmune
Syndrome Induced by Silicone.”
For the BIA-ALCL: “anaplastic large cell lymphoma,” “breast implant-associated with
anaplastic large cell lymphoma,” and “silicone implant complications.”
In the other stages, the selection criteria were equivalent:
Stage III aimed to define the search portals for articles, the database being the
electronic sites of PubMed, Science Direct, and Periódicos Capes.
Step IV constituted the selection phase of the articles found, which was performed
by reading the title, abstract, and, in cases of doubt or interest, the complete reading
of the journal to answer the research problem.
Finally, in step V, the results were analyzed, via mental maps and other instruments,
to generate the present results and discussions.
RESULTS
In the end, 968 articles were analyzed, of which 20 met the abovementioned criteria,
intending to address ASIA and BIA-ALCL as possible complications of breast implants.
Of the 19 articles selected, 9 reported the pathophysiology and symptomatology of
ASIA, 7 commented on BIA-ALCL, and 4 on general aspects of complications caused by
silicone implants.
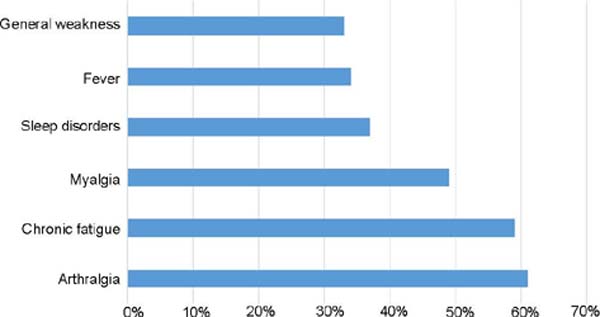
Concerning the main complications found, arthralgia and chronic fatigue were the main
symptoms found in ASIA (Figure 2).
Figure 2 - Main symptoms found in ASIA. Graph available from Watad A, Rosenberg V, Tiosano S,
Cohen Tervaert J W, Yavne Y, Shoenfeld Y, et al. Silicone breast implants and the
risk of autoimmune/rheumatic disorders: a real-world analysis. Int J Epidemiol. 2018;47(6):1846-54
3.
Figure 2 - Main symptoms found in ASIA. Graph available from Watad A, Rosenberg V, Tiosano S,
Cohen Tervaert J W, Yavne Y, Shoenfeld Y, et al. Silicone breast implants and the
risk of autoimmune/rheumatic disorders: a real-world analysis. Int J Epidemiol. 2018;47(6):1846-54
3.
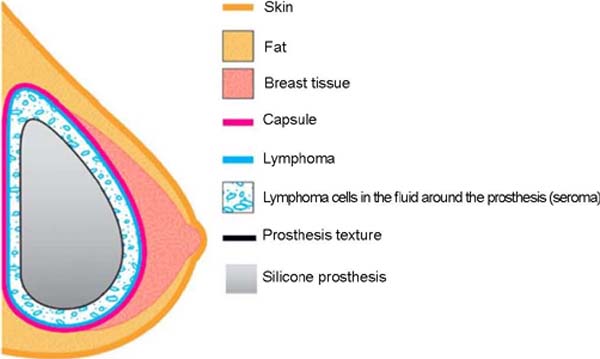
In the pathophysiology of the involvement causing BIA-ALCL, the most accepted theory
is that breast implants with a larger surface area would form a greater biofilm due
to greater bacterial adhesion, generating more prominent chronic inflammation and
triggering the malignant transformation of the breasts. T cells, the main symptom
being late stroke (Figure 3).
DISCUSSION
Adjuvant-induced autoimmune/inflammatory syndrome
ASIA is a disease characterized by chronic pain and joint manifestations, among other
symptoms. Currently, it is in evidence due to its correlation with silicone implants,
which are increasingly widespread in society and are in high demand by young and adult
women.
Since the 1990s, prostheses have been the subject of discussion, especially due to
the appearance of a new “disease” related to implants called siliconosis or “silicone
reactive disease.” However, this syndrome gained great repercussions only about a
decade ago when it was described by Schoenfeld & Agmon-Levin in a study published
in 2011 called “’ASIA’ – autoimmune/inflammatory syndrome induced by adjuvants”9.
Watad et al.10, in their work on Shoenfeld’s syndrome, refer that the onset of autoimmune conditions
results from the interaction of a genetic predisposition and exposure to environmental
factors, resulting in an organism’s autoimmunity process.
Vera-Lastra et al.11 reported in one of their articles that the silicone present in the prosthesis gel
can oxidize into silica, increasing the activity of the immune response. The mechanism
used to activate the immune and adaptive system consists of Th1 and Th17 activation
and the release of interleukin 17, which will cause a response that will stimulate
fibroblasts to produce fibrosis. All this cytokine modulation mechanism explains one
of the reasons for causing capsular contracture in autoimmune diseases. According
to Colaris et al.12, this capsular contracture can also be seen as one of the most frequent complications,
almost always related to a deficient humoral system.
Several physicians and researchers began correlating the symptoms women presented
with their respective silicone implants. For Pavlov-Dolijanovic & Vujasinovic Stupar5,
unexplained symptoms such as fatigue, neurasthenia, myalgia, arthralgia, morning stiffness,
and night sweats are present in more than 60% of women. Furthermore, some patients
also had cognitive problems, dermatological and gastrointestinal symptoms, alopecia,
and sleep disorders.
Watad et al.3 mention that arthralgia was seen in approximately 61% of all cases. Chronic fatigue
was present in 59% of the total. Myalgia in 49% of cases. Sleep disorders in 37%.
General weakness presented in 33% and sicca symptoms in 18%. Fever was seen in 34%
of patients, arthritis in 29%, and neurological manifestations in 26%.
Balk et al.13 demonstrate that, in addition to the symptoms already mentioned, there is the possibility
of association of connective tissue diseases such as Raynaud’s Phenomenon, dermatomyositis,
scleroderma, and polymyositis with the silicone implant.
Watad et al.10 point out that in work by Cohen et al., it is reported that 30-50% of women who develop
ASIA have Raynauld’s phenomenon. In addition, most patients had antinuclear antibodies
and other unspecified antibodies. The authors also point out that the implants act
as adjuvants to induce local and systemic reactions triggered by macrophages and T
cells that will produce antibodies and systemic symptoms.
Another fact mentioned is that about 14 months after removing the silicone implant,
all the symptoms improved or regressed, indicating a regression of the autoimmune
reaction. About 63% of women who underwent surgery to extract the silicone felt improvement
in symptoms such as myalgia, arthralgia, fatigue, and neurological symptoms.
There is still great divergence in the literature about the time of appearance of
these symptoms. Studies show that symptoms begin from 1 month to 39 years after silicone
implant surgery. The work published by Watad et al.3 states that the symptoms appear in the interval of 1 week to 60 months after implant
surgery. Fenoglio et al.14 suggest that the time interval between prosthesis placement and the onset of symptoms
is approximately 2 years.
Regarding the diagnosis of ASIA, according to the study published by Schoenfeld &
Agmon-Levin9, the diagnosis should be based on major and minor criteria. It is organized as follows:
either fulfilling 2 major criteria or a major and a minor criterion based on Chart 2, which was taken from the same study published in 2011.
Chart 2 - Adapted from: Cohen Tervaert J W. Autoinflammatory/autoimmunity syndrome induced by
adjuvants (ASIA; Shoenfeld's syndrome): A new flame. Autoimmun Rev. 2018;17(12):1259-64
2.
| Major criteria: |
| • Exposure to an external stimulus (infection, vaccine, silicone, adjuvant) before
clinical manifestations
|
|
• Appearance of one of the following clinical manifestations:
- Myalgia, myositis, or muscle weakness;
- Arthralgia and/or arthritis;
- Chronic fatigue, restless sleep, or sleep disturbances;
- Neurological manifestations (especially associated with demyelination);
- Cognitive impairment, memory loss;
- Fever, dry mouth.
|
| • Removal of the initiating agent induces improvement |
| • Typical biopsy of involved organs |
| Minor criteria: |
| • Appearance of autoantibodies directed against the suspected adjuvant |
| • Other clinical manifestations (e.g., irritable bowel syndrome) |
| • Specific HLA (e.g., HLA DRB1, HLA DQB1) |
| • Emergence of an autoimmune disease (e.g., multiple sclerosis, systemic sclerosis) |
| For the diagnosis of ASIA, at least two major or one major and two minor criteria |
Chart 2 - Adapted from: Cohen Tervaert J W. Autoinflammatory/autoimmunity syndrome induced by
adjuvants (ASIA; Shoenfeld's syndrome): A new flame. Autoimmun Rev. 2018;17(12):1259-64
2.
Anaplastic Large Cell Lymphoma
Another disease with increased incidence due to the increase in breast implants, in
addition to having a better diagnosis, is BIA-ALCL, which is directly related to silicone,
especially those with a textured capsule10.
The pathogenesis of the disease is still not very clear; however, a higher rate of
BIA-ALCL development was associated with textured capsule implants (this textured
capsule was created in the 60s as an alternative to reduce cases of capsular contracture,
but the results are contradictory).
One of the theories on the development of lymphoma is based on forming a subclinical
biofilm, capsular contracture, repetitive trauma, genetic predisposition or autoimmune
etiology relating to ASIA, and immune response to silicone components themselves were
also considered7. According to the 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)15, no confirmed disease cases exist in patients with non-textured prostheses.
There are two courses of disease, one being in situ, where there is a disease in the
effusion or the inner wall of the capsule; this course usually does not have a mass
and can be confused with a benign seroma. There is also an infiltrative course, presenting
with a palpable mass and affecting underlying tissues; this presentation has a worse
prognosis (40% mortality in 2 years). Both can occur with lymph node alteration, and
there are also cases of lymph node alteration without other symptoms7.
The symptomatology of BIA-ALCL presents itself as pain and breast asymmetry with a
palpable mass; however, its clinic can be variable, with the presence of periprosthetic
effusion, effusion with mass, isolated mass, with or without seroma, or only lymph
node involvement. The most common presentation is late effusion (48%-70% of cases),
which can occur nine years after implantation7. Therefore, any presentation of late effusion with rapid evolution that cannot be
explained by infection or trauma should lead to suspicion of BIA-ALCL16.
Any alteration in the silicone prosthesis must be investigated, always starting with
a mammogram to look for a mass, liquid collection, or lymph node enlargement – if
not conclusive, an MRI can be requested. A biopsy or fine needle aspiration should
be performed when a seroma or mass is found. Other investigations can be performed
are CD30 dosage and anaplastic kinase lymphoma, the first being positive and the second
negative.
BIA-ALCL can appear on average 8-10 years after the implant procedure16, and when diagnosed, it is important to remove the complete capsule as soon as possible
– a procedure performed by the plastic surgeon himself.
The cure of BIA-ALCL depends on the surgical removal of the prosthesis, with the capsule,
and in infiltrative cases of the affected underlying tissue (including lymph nodes
according to the staging). When correctly removed, there is a recurrence rate of 6%-11%,
0% for patients staged in T1 and 2, and 14.3% for stage 4. Survival is not affected
by the use of postoperative chemotherapy, and the removal of the contralateral prosthesis
should be discussed (4.6% of the cases are bilateral).
The placement of a new prosthesis after identifying the BIA-ALCL7 is contraindicated. Adjuvant treatments such as radiotherapy, chemotherapy, and stem
cell transplants have proven very effective and should be combined with surgery15.
Outpatient follow-up after healing is important; reassessed every 3-6 months for two
years if there is no sign of recurrence. In the evaluations, tomography or PET scan16 can be performed.
CONCLUSION
Complications secondary to silicone implants are being increasingly studied and disseminated
due to the increased prevalence of this surgery. As mentioned in this article, ASIA,
and BIA-ALCL are some of the main complications resulting from this procedure. However,
despite its pathophysiology being well elucidated in the literature, the symptomatology
and the onset of symptoms still do not have a standard since several pieces of literature
diverge in this sense, in addition to some authors placing a very high time interval
for the appearance initial.
Concerning ASIA, it was noted that the most prevalent symptom is arthralgia, followed
by chronic fatigue, resulting from the activation of the Th1 and Th17 immune system
and the release of interleukin 17 – resulting in fibrosis from fibroblasts – since
the silicone present in the prosthesis gel can oxidize into silica, causing an increase
in immune activity.
In BIA-ALCL, the most prevalent symptom is late effusion, with a period of symptomatic
onset years after surgery – approximately 9 years – after which the evolution occurs
acutely. The pathophysiology of this complication occurs mainly with the development
of seroma, being directly related to textured capsule implants, in addition to being
related to the type of breast implant, genetic predisposition, and the formation of
a subclinical biofilm, being necessary to evaluate the quality of the material used,
since it can predispose to the formation of this biofilm and lead to complications.
Therefore, silicone implants can cause complications that affect patients’ quality
of life, and attention should be paid to initial symptoms and seeking medical help
in case of any intercurrence, regardless of the time interval, since the complications
above can occur many years after the surgery.
1. Universidade da Região de Joinville, Joinville, SC, Brazil.
2. Sociedade Brasileira de Cirurgia Plástica, Curitiba, PR, Brazil.
Corresponding author: Natalia Silva Zahdi Rua Paulo Malschitzki, 10, Zona Industrial Norte, Joinville, SC, Brazil Zip code:
89201-972 E-mail: nataliazahdi@gmail.com