INTRODUCTION
Breast implants are routinely used in cosmetic and restorative breast plastic surgery;
according to the American Society of Plastic Surgery, in 2016, 290,467 implants for
aesthetic purposes and 109,256 implants for repair procedures1. Although widely used, there is a risk of capsular contracture in 10.6% of operated
cases2. However, several patients should undergo radiotherapy as local breast cancer treatment
when performing reconstructive surgery, such as breast reconstruction. In these cases,
the prevalence of this complication is greater than 40%, and the need for removal
of the prosthesis is 15%2.
When inert materials, such as silicone are implanted in the human body, the body forms
a connective tissue capsule around the implant to isolate it from adjacent tissues.
In some cases, this envelope becomes fibrotic due to trauma, infection, and other
unknown causes (capsular contracture). Depending on the degree of fibrosis may cause
aesthetic deformity, pain and inflammation of the implant site3. Therefore, when the prosthesis is encapsulated, the treatment should be surgical
with the removal of the prosthesis and replacement by another prosthesis in another
anatomical plane, for example, if the prosthesis is placed above the pectoral muscle
and there is a contracture, it should be replaced by another prosthesis implanted
below the muscle. This procedure aims to avoid recurrence of contracture 4,5.
For this reason, several techniques have been studied to prevent the occurrence of
capsular contractures, such as the use of textured implants, implantation of the prosthesis
in a submuscular pocket4, use of medications such as corticosteroids, cyclosporine5 and antileukotries6,7. All these therapeutic alternatives showed variable results without composing clinical
evidence.
Bastos et al. (2012)8 showed positive results of using Montelukast, an antagonist of leukotrienes receptors,
to prevent capsular contracture.
OBJECTIVE
This study will analyze the effect of Montelukast on animal models of radio-induced
capsular contracture. Therefore, this study aimed to analyze the effect of the antileukotriene
drug (Montelukast®) on capsular contracture formation in rats with silicone implants who underwent radiotherapy
within two months.
METHODS
Twenty Wistar rats were analyzed, with weights ranging from 250-300g. All animals
were kept in a bioterium with controlled temperature between 22-23ºC, day/night cycle
of 12.12h, water and food ad libitum. This study followed the national rules for the
standardization of studies on laboratory animals (CONCEA) and was approved by the
Ethics Committee on Animal Use of the Faculty of Medicine of the University of São
Paulo (CEUA-FMUSP) under registration 067/2017.
Surgical procedure
The animals were anesthetized by a solution composed of 100mg/kg of weight of ketamine
hydrochloride (Ketamin®, Cristália, Brazil) and 10mg/kg of xylazine hydrochloride (Rompun®, Bayer, Brazil) by intraperitoneal route.
After intraperitoneal anesthesia, the animals were positioned in ventral decubitus.
Trichotomy was then performed in an area of 6cm2 in the region of the midline of the spine just below the scapulae. Topical chlorhexidine
was used for antisepsis in this region.
A 2cm horizontal incision was performed using a 15-inch blade scalpel. Pocket dissection
of 6 cm2 below the plane of the panniculus carnosus of the animal and hemostasis with an electric
scalpel. Before performing the closing of the pocket was implanted a 2x2 cm silicone
block. And then the animals were randomized into two groups: control (n=10), cream
injection around the silicone block using 1ml of 0.09% saline solution; antileukotriene
group (n=10), tissue injection around the silicone block using 1ml of antileukotriene
(Singulair®, Merck Sharp &Dohme, Campinas, Brazil) diluted in 0.9% saline solution.
Radiotherapy
All animals were submitted in the immediate postoperative period to a radiotherapy
session. The radiation was made a specific irradiator for small animals (Rad Source
RS2000, Quastar™), with an X-ray of 160KV of 25mAs.
The animals were positioned in lateral decubitus contained by an immobilizer that
allowed the emission of rays in the tangential plane as used in patients. A 5mm lead
plate was also inserted that had a window for the irradiation of the animal, ensuring
treatment only in the desired region. A single dose of 10Gy was used in two opposite
fields (5Gy per field) with a 6Gy/min card.
Histological analysis
After two months, the animals were euthanized by intraperitoneal injection of ketamine
hydrochloride (Ketamin®, Cristália, Brazil) at a dose of 180mg/kg of weight, associated with xylazine (Rompun®, Bayer, Brazil) at a dose of 15mg/kg and then placed in a 100% CO chamber.
Samples of the capsule were collected around the silicone block through an incision
in the back of the animals. Part of the samples was fixed with formalin 4% for 24h
and then colored with hematoxylin-eosin (HE) and picrosirius for microscopy analysis
(Nikon eclipse E600®, Japan) in magnification of 200 and 400 times.
The hematoxylin-eosin-stained slides analyzed the presence of inflammatory cells and
vascular density (arteriole count) in 10 fields along the histological sections. The
slides stained by the picrosirius method graphically demonstrated collagen deposition
along with the capsule around the silicone block.
Gene expression analysis
Total RNA extraction
Panniculus carnosus samples were macerated using the Tissue Lyser LT device (Qiagen,
Germantown, USA). 1.0ml Trizol® (Invitrogen-Life Technologies, Carlsbad, USA) and
stainless-steel beads were added to microcentrifuge tubes with samples. Fragmentation
was performed for 6 minutes at 50Hz.
After the removal of the spheres, 0.2 ml of chloroform (Merck) was added. The samples
were centrifuged for 15 minutes at 12,000rpm at 4ºC. After centrifugation, the aqueous
phase was transferred to a new microcentrifuge tube, and 0.5ml of icy isopropyl alcohol
(Merck) was added for RNA precipitation. The samples were incubated at room temperature
for 10 minutes and then centrifuged at 12,000rpm for 10 minutes at 4ºC. The supernatant
was discarded, and the RNA precipitate was washed with 1.0ml of 75% ethanol. The RNA
pellet was resuspended in 50 to 100µL of sterile ultrapure water free of DNase/RNase
(Invitrogen-Life Technologies, Carlsbad, USA).
The concentration of the extracted RNAs was determined in a NanoDrop(tm) ND-1000 spectrophotometer
(NanoDrop Technologies, Inc., Wilmington, USA). The degree of purity was evaluated
using the 260/280nm ratio, using only RNAs whose ratio was ≥1.8. To analyze the integrity
of the RNAs, agarose gel electrophoresis was performed to verify the 28S and 18S bands.
The extracted RNAs were stored at -80°C until use.
cDNA Synthesis
For cDNA synthesis from the total RNA, the High Capacity RNA-to-cDNA (AppliedBiosystems)
kit was used in GeneAmp 2400 thermocycler (Applied Biosystems). In the final volume
of 20µL: 1.0µL of enzyme mix; 10.0µL of RT buffer; 20µL q.s. of sterile ultrapure
water free of DNase/RNase and total RNA (500ng). For reaction and reaction stoppage,
the tubes were incubated at 37ºC for 60 minutes and 95ºC for 5 minutes, respectively.
cDNA samples were stored at -20ºC until use.
Real-time polymerase chain reaction (qRT-PCR)
The analysis of the expression of the mRNA levels of the genes of interest was performed
by qRT-PCR in the StepOnePlusTM (AppliedBiosystems) thermocycler with the TaqMan® Gene Expression Assays (Applied Biosystems) system.
Probes and primers for the Genes C5AR1 (Rn02134203), ICAM 1 (Rn 00564227), NOS2 (Rn
00561646), VEGF (Rn 01511602) and the endogenous control ACTB (Rn 00667869) were acquired
from the list of inventoried assays of the company Applied Biosystems.
QRT-PCR was performed in duplicate for each sample using: 10.0µL TaqMan® Universal
Master Mix II 2X; 1µL TaqMan® Gene Expression Assay 20X and 4µL of diluted cDNA (dilution
1:5) in the final volume of 20µL, in plates of 96 wells covered with an optical sealant.
The reaction conditions were 50ºC for 2 minutes, 95 for 10 minutes, followed by 40
cycles at 95ºC for 15 seconds and 60ºC for 1 minute.
For the calculation of the expression level of each target gene, GenEx Standard 6.1
(MultiD Analyses AB)software was used, which uses the 2-delta delta Ct method for
relative quantification, where Ct (threshold cycle) is the real-time PCR cycle, in
which amplification reaches the logarithmic phase, where delta Ct is the difference
of expression between the target gene and endogenous control of a given sample, and
delta delta Ct corresponds to the difference between the delta Ct of the sample and
the delta Ct of the control.
Statistical analysis
The variables were submitted to descriptive analysis. After checking the distribution
and variances of the data, the two groups were compared using the Wilcoxon rank-sum
test (non-normal distribution), considering an alpha p of 0.05. Stata software version
14 (StataCorp., 2015) was used. Stata Statistical Software: Release 14. College Station,
TX: StataCorp LP, USA).
RESULTS
Three animals in the control group and one in the AL group presented dehiscence and
extrusion of the silicone block after four weeks of irradiation.
Histological analysis
The analysis by the histomorphometry method showed no difference in the number of
inflammatory cells. However, there was a decrease in vascular density in the group
treated with AL when compared to the control group (55.4±30.0 (AL) vs. 81.8±26.7 (control);
p=0.05) (Table 1 and Figure 1).
Table 1 - Analysis by histomorphometry refers to inflammatory cells and vascular density in
the control and AL groups.
| |
Control (n=7) (average±SD) |
Antileukotriene (n=9) (average±SD) |
| Inflammatory cells |
8.25±5.9 |
4.4±4.0 |
| Vascular density |
81.8±26.7 |
55.4±30.0 |
Table 1 - Analysis by histomorphometry refers to inflammatory cells and vascular density in
the control and AL groups.
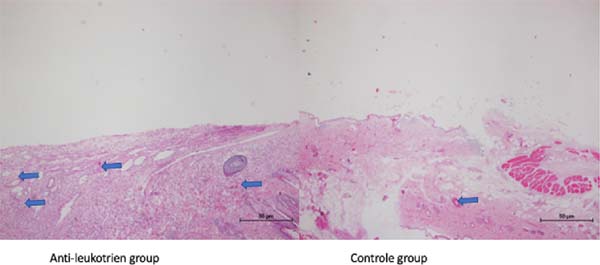
Figure 1 - Slides colored by HE method, samples by groups. The blue arrows correspond to arterioles
along with the capsule.
Figure 1 - Slides colored by HE method, samples by groups. The blue arrows correspond to arterioles
along with the capsule.
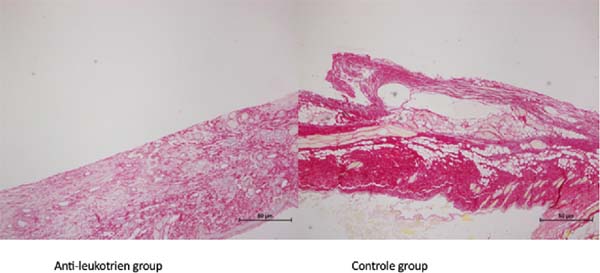
The slides colored by picrosirius showed a higher amount of collagen in the control
group (Figure 2).
Figure 2 - Picrosirius staining showed the density of collagen fibers in the control group when
compared to the AL group.
Figure 2 - Picrosirius staining showed the density of collagen fibers in the control group when
compared to the AL group.
Analysis of gene expression of biomarkers (iNOS, VEGF-a, MMP-9)
The AL group showed a lower amount of VEGF-a when compared to the control group (0.02±0.001
vs. 0.34±0.1, respectively, p=0.04) (Table 2).
Table 2 - Gene expression of iNos, VEGF-a, ICAM-1 and MMP-9 in the control and AL groups.
| |
Control (n=7)(average±SD) |
Antileukotriene (n=9) (average±SD) |
| iNOS |
0.013±0.01 |
0.005±0.001 |
| VEGF-a |
0.34±0.1 |
0.02±0.001 |
| MMP-9 |
0.36±0.2 |
0.30±0.1 |
Table 2 - Gene expression of iNos, VEGF-a, ICAM-1 and MMP-9 in the control and AL groups.
DISCUSSION
Leukotrienes are lipids (eicosanoid) belonging to the family of inflammation-mediating
substances. Act on smooth muscle contraction (bronchoconstriction) and increasing
microvascular permeability perpetuating the inflammatory reaction9.
Antileukotrienes, therefore, have the function of reducing and preventing smooth muscle
contraction and reducing vascular permeability. Although the mechanism of action has
not yet been elucidated, several studies have demonstrated the beneficial effect of
antileukotrienes in inhibiting or minimizing capsular contracture after breast alloplastic
implants6-8,10.
Our hypothesis was to analyze the effect of Al on the local inflammatory reaction
(decrease in late neoangiogenesis and decrease in inflammatory cells) and inhibition
of fibrosis formation. To enhance the results of this study and to pet the clinical
situation of treatment adjunct to breast cancer reconstruction, all animals received
a dose of irradiation immediately after surgery.
This study demonstrated a 50% decrease in inflammatory cell count in the Al group
(4.4±4.0) compared to the control group (8.25±5.9). Although it did not reach significance,
there is a tendency to observe less inflammatory reaction in the AL group. In the
histological analysis regarding vascular density, this study demonstrated a lower
count of neoformed vessels in the AL group when purchasing with the control group
(55.4±30.0 vs. 81.8±26.7, respectively; p=0.05). These two findings suggest decreased
chronic inflammation in the silicone implant capsule. Kang et al. (2015) 11 analyzed the use of Zafirlukast in the formation of silicone implant capsules in
rabbits. These authors demonstrated a similar result to our study; animals treated
with Al showed decreased capsular contracture after two months of follow-up. Still,
Kang et al. (2015) 11 also analyzed the inflammatory reaction around the silicone implant. Again, this
group demonstrated a decrease in macrophages, as presented in our study.
Still, Kang et al. (2015) 11 studied an intermediate outcome measuring the gene expression of myofibroblasts.
The authors showed a decrease in the expression of the myofibroblast gene in the AL-treated
group compared to the control group. Based on these literature findings, our group
explored other pathways responsible for the mechanism of action of AL.
The expression of the iNOS gene was higher in the control group than in the AL group
(0.013±0.01 and 0.005±0.001, respectively) (p=0.08), but in this study, this difference
showed a tendency to significance. The action of nitric oxide inducible synthetase
(iNOS)refers to an enzyme in the inflammatory response mediated by macrophages. And,
consequently, the increase in nitric oxide (NO) produced by macrophages induces a
pro-inflammatory state. Therefore, the decrease in gene expression in the AL group
suggests an anti-inflammatory effect on the silicone implant capsule. As demonstrated
by McNeill et al. (2015)12. According to this study, the dissection of macrophages, according to the free radical
pathway, demonstrated a decrease in the inflammatory process due to the inhibition
of iNOS11,12.
Another biomarker studied by McNeill et al. (2015) 12 was VEFG-a as a possible factor contributing to the mechanism of action to the anti-inflammatory
effect of AL. In our study, the gene expression of VEGF-a decreased in the group treated
with AL compared to the control group. Reflecting on this outcome, the decrease in
the long-term neoangiogenesis marker (2 months) may demonstrate the transition of
phenotype 1 macrophages to phenotype 2; there is the resolution of the inflammatory
process13. Another intermediate outcome analyzed in this study was the gene expression of MMP-9,
which corresponds to gelatinase metabolism (extracellular matrix component). In addition,
several authors have analyzed other metalloproteinases, intending to study collagen
metabolism in tissue regeneration14,15.
This study found no difference in gelatinase (MMP-9) between groups. However, this
study presented some limitations: the anatomical structure of the mine model adopted
in this study presented some differences (panniculus carnosus) concerning humans.
CONCLUSION
This study suggests that the treatment with AL decreases angiogenesis in animals submitted
to silicone implants and underwent radiotherapy
ACKNOWLEDGMENTS
This study would not be possible without the collaboration of Mrs. Silvana Aparecida
Biagion, Mrs. Edna Maria Rodrigues dos Santos, Mrs. Roqueline Alves Lago and Mr. Bruno
Valério do Rosário.
REFERENCES
1. American Society of Plastic Surgeons (ASPS). 2016 National plastic surgery statistics
- cosmetic & reconstructive procedure trends [Internet]. Arlington Heights: ASPS;
2017; [acesso em 2017 Mai 15]. Disponível em: https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/2016-plastic-surgery-statistics-report.pdf
2. Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review
of the literature. Plast Reconstr Surg. 2009 Ago;124(2):395-408.
3. Headon H, Kasem A, Mokbel K. Capsular contracture after breast augmentation: an update
for clinical practice. Arch Plast Surg. 2015 Set;42(5):532-43. DOI: https://doi.org/10.5999/aps.2015.42.5.532
4. Steiert AE, Boyce M, Sorg H. Capsular contracture by silicone breast implants: possible
causes, biocompatibility, and prophylactic strategies. Medical Devices. 2013 Jun;6:211-8.
DOI: https://doi.org/10.2147/MDER.S49522
5. Collis N, Coleman D, Foo ITH, Sharpe DT. Ten-year review of a prospective randomized
controlled trial of textured versus smooth subglandular silicone gel breast implants.
Plast Reconstr Surg. 2000 Set;106(4):786-91.
6. Miller AS, Tarpley SK, Willard VV, Reynolds GD. Alteration of fibrous capsule formation
by use of immunomodulation. Aesthet Surg J. 1998 Set/Out;18(5):346-52.
7. Schlesinger SL, Ellenbogen R, Desvigne MN, Svehlak S, Heck R. Zafirlukast (Accolate):
a new treatment of a difficult problem. Aesthet Surg J. 2002 Jul;22(4):329-36.
8. Bastos ÉM, Sabino Neto M, Garcia ÉB, Veiga DF, Han YA, Denadai R, et al. Effect of
zafirlukast on capsular contracture around silicone implants in rats. Acta Cir Bras.
2012;27(1):1-6. DOI: https://dx.doi.org/10.1590/S0102-86502012000100001
9. Peters-Golden MD, Henderson Junior WR. Leukotrienes. N Engl J Med. 2007 Nov;357(18):1841-54.
DOI: https://doi.org/10.1056/NEJMra071371
10. Vieira VJ, D'Acampora A, Neves FS, Mendes PR, Vasconcellos ZA, Neves RD, et al. Capsular
contracture in silicone breast implants: insights from rat models. An Acad Bras Ciênc.
2016 Set;88(3):1459-70. DOI: https://doi.org/10.1590/0001-3765201620150874
11. Kang SH, Shin KC, Kim WS, Bae TH, Kim HK, Kim MK. The preventive effect of topical
zafirlukast instillation for peri-implant capsule formation in rabbits. Arch Plast
Surg. 2015 Mar;42(2):179-85. DOI: https://doi.org/10.5999/aps.2015.42.2.179
12. McNeill E, Crabtree MJ, Sahgal N, Patel J, Chuaiphichai S, Iqbal AJ, et al. Regulation
of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements
for tetrahydrobiopterin in NRF2 activation. Free Radic Biol Med. 2015 Feb;79:206-16.
DOI: https://doi.org/10.1016/j.freeradbiomed.2014.10.575
13. Butler CT, Reynolds AL, Tosetto M, Dillon ET, Guiry PJ, Cagney G, et al. A quininib
analogue and cysteinyl leukotriene receptor antagonist inhibits vascular endothelial
growth factor (VEGF)-independent angiogenesis and exerts an additive antiangiogenic
response with bevacizumab. J Biol Chem. 2017 Mar;292(9):3552-67. DOI: https://doi.org/10.1074/jbc.M116.747766
14. Ulrich D, Ulrich F, Pallua N, Eisenmann-Klein M. Effect of tissue inhibitors of metalloproteinases
and matrix metalloproteinases on capsular formation around smooth and textured silicone
gel implants. Aesthetic Plast Surg. 2009 Jul;33(4):555-62. DOI: https://doi.org/10.1007/s00266-009-9335-y
15. Chen CZ, Raghunath M. Focus on collagen: in vitro systems to study fibrogenesis and
antifibrosis - state of the art. Fibrogenesis Tissue Repair. 2009 Dec;2:7. DOI: https://doi.org/10.1186/1755-1536-2-7
1. University of São Paulo, São Paulo, SP, Brazil.
Corresponding author: Cristina Pires Camargo, Avenida Brigadeiro Luis Antonio, nº 4161 - Jardim Paulista, São Paulo, SP, Brazil,
Zip Code 01402-002. E-mail: cristinacamargo@usp.br
Article received: November 02, 2020.
Article accepted: April 19, 2021.
Conflicts of interest: none.