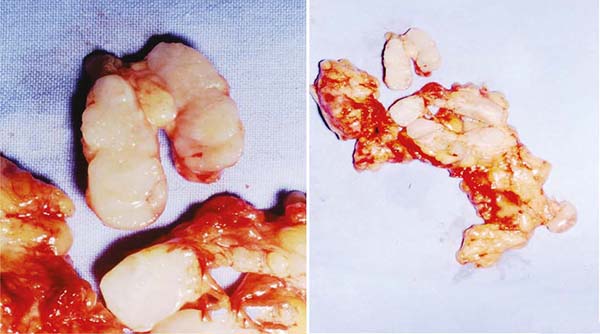
Silicone breast implants came from Cronin and Gerow’s idea in 19611, observing a more or less full blood bag that had a consistency similar to the breast.
They developed a prototype and implanted it for the first time in the dog Esmeralda,
with the participation of the resident Thomas Biggs, who took care of her, as he declares
in congresses. Biggs was in Brazil at congresses more than 40 times, the first in
São José do Rio Preto at the invitation of Dr. Melchiades Cardoso de Oliveira, to
participate in a Plastic Surgery Day held in 1971. He performed three surgeries: a
rhytidoplasty, a resection of ameloblastoma taking a half jaw, reconstructed with
an iliac crest bone graft, and a genitals correction of an intersex child; an excellent
surgeon.
The first implants consisted of a low cohesiveness and hardness gel, with not very
thin capsules with a smooth surface and Dacron patches at the base, which adhered
to the pectoral aponeurosis muscle fixing the implant in its position so it would
not change with time. It was their first generation that lasted from 1963 to 1972
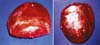
(Figure 1).
Figure 1 - First implants designed by Cronin and Gerow. Photo obtained from Mama-book, page 103.
Editor of volume 5: James C. Grotting, Editor: Peter C. Neligan. Elsevier Saunders,
third edition- 2015.
Figure 1 - First implants designed by Cronin and Gerow. Photo obtained from Mama-book, page 103.
Editor of volume 5: James C. Grotting, Editor: Peter C. Neligan. Elsevier Saunders,
third edition- 2015.
Patients’ problems with hypomastias seemed to be solved, but it was not exactly what
would happen over time as it was just beginning. These patches were abolished from
the second generation’s industrialization since it was observed that they were not
necessary to keep the implants in their positions. Furthermore, they were not exempt
from complications, producing a foreign body reaction more intense than the implant
and contributing to its deformation. They remained similar to the first, without Dacron,
between 1973 and 1980. In 1974, we had first contact with one of them.
Many of these implants filled with silicone gel became hard with capsular contracture
when reaching around one year after the operation. Numerous publications that appeared
since 1974 and others afterward demonstrated the attempt to prevent these contractures
by reducing fibrosis; some of them using corticosteroids to reduce the inflammatory
reaction around the implant2,3; others researched the causes of contractures4,5, and there was a demonstration that hematomas cause higher capsular contractures6; in addition to a classification of their intensity7. If they were hard, the industry started making implants with the softest gel, most
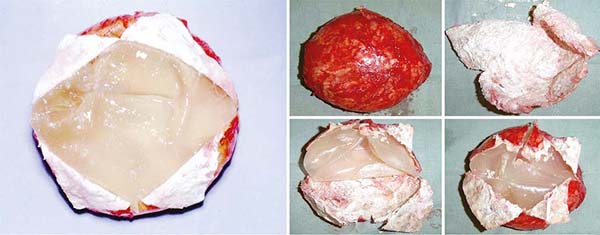
delicate capsules, and even half-empty (Figure 2).
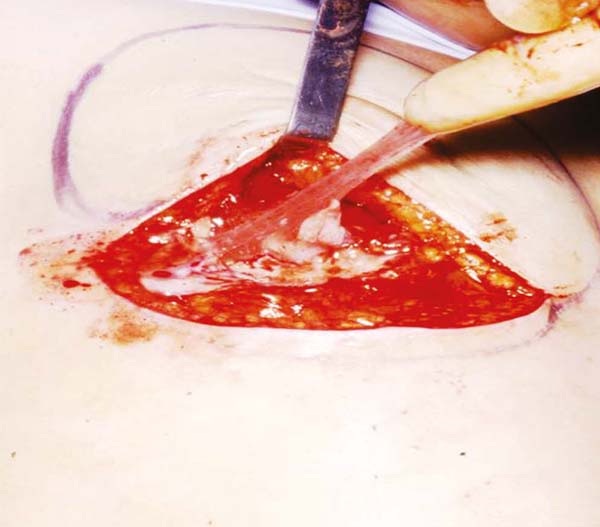
Figure 2 - The implant is half empty with folds and “exuding” over the surgical field.
Figure 2 - The implant is half empty with folds and “exuding” over the surgical field.
The “logical” deduction was: if they become hard, make the implants softer and more
delicate with thinner capsules, and the problem of their hardness in the late postoperative
period would be solved; logically to remain that way over time when deployed. The
reverse way of the real is necessary. This was the third generation, approximately
between the 1980s and the 1990s. Nevertheless, that was not what happened. They got
even harder and more quickly, in addition to bringing a series of consequences. At
that time, there was a lack of understanding of capsular contracture’s biomechanics,
associated with a foreign body reaction8 already known, but not recognized.
Leakage of the silicone gel, which exceeded the implant capsules’ barrier and the
fibrous layer to the underlying breast tissues, was observed very frequently, even
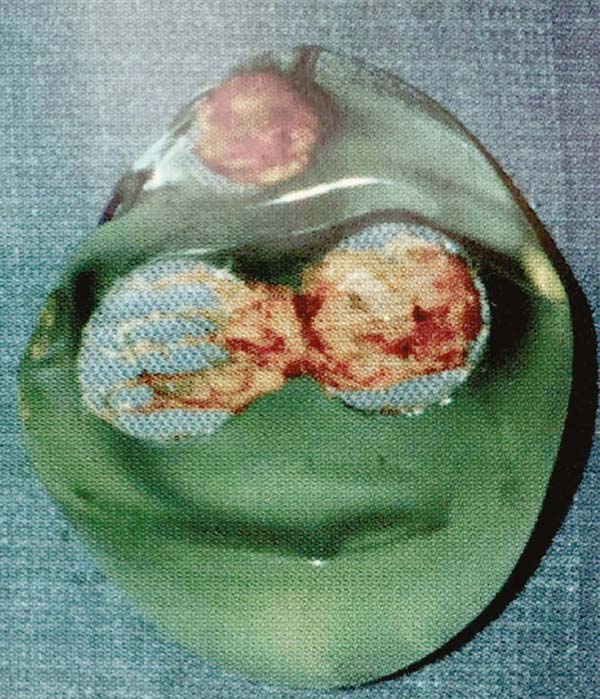
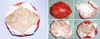
without rupture, creating numerous nodules in new mini capsules (Figures 3A and 3B). With a longer time, the axillary lymph nodes migrated and impregnated (Figure 4), often confused with tumor metastases (Figures 5A and 5B).
Figure 3 - Diffuse nodulations encapsulated in mini capsules after implant silicone leakage.
Figure 3 - Diffuse nodulations encapsulated in mini capsules after implant silicone leakage.
Figure 4 - Lymph nodes impregnated with extravasated silicone gel and migrated to the armpits.
Figure 4 - Lymph nodes impregnated with extravasated silicone gel and migrated to the armpits.
Figure 5 - Axillary nodes invaded by tumor metastases and silicone gel leaking from the implants
Figure 5 - Axillary nodes invaded by tumor metastases and silicone gel leaking from the implants
There was no magnetic resonance imaging and guided-needle biopsy (GNB). Furthermore,
if these implants were placed on a surgical field, when they were removed, they left
an “oily” mark resulting from the leakage of the gel that crossed the barrier of the
capsule that surrounded it. In several congresses in Brazil, a photo projected by
Prof. Dr. Andrews J. M.9 (Figure 2) demonstrating this overflow. The smooth, thin capsule broke easily and, in the long
run, often dissolved, disappeared, or confused with the inner gel (Figure 6).
Figure 6 - Implants with the dissolved capsule merging with the inner gel.
Figure 6 - Implants with the dissolved capsule merging with the inner gel.
The hardness of the silicone gel is measured in centistokes10. The silicone gel is surrounded by a layer of more rigid laminated silicone, with
more centistokes, and the natural solvent of the hardest silicone is the softest and
most liquid. The softer or more liquid, the greater the dissolution capacity of the
harder silicone (elastomer). Then, the inner gel dissolves the capsule causing it,
in some cases, to disappear completely10.
If the understanding of this process and the biological reaction of a foreign body
was lacking, which was not observed with greater attention and depth at that moment,
we will analyze it. It is immutable and inexorable; it is a natural law like countless
natural laws of physics, mathematics, and biology.
Around a foreign body implanted in human tissues, there is a biological tendency to
isolate it, and a classic healing reaction begins, involving inflammation, fibroplasia,
and maturation, with deposition of fibroblasts/myofibroblasts/fibrocytes/collagen
on its surface, making a fibrous “protective” capsule, from the foreign body and the
organism that houses it. This is what happens with breast implants11.
If it is contaminated, it tends to be eliminated by the route closest to the outside
environment; that is, it is a “biological intelligence” of self-protection. Moreover,
this occurs by destroying tissues in this path until the skin opens and expels it.
In general, the weak point is the scar resulting from the surgery and is not resolved
by trying to suture the skin that has opened. It will open as many times as it is
sutured. It can be through the skin, but in other foreign bodies, it can happen through
the intestinal loops or airways; it is biology that chooses the easiest and shortest
path.
The longer the process lasts until elimination, the more intense and thicker the fibrous
capsule is required. Furthermore, the major fibrosis will be at the opposite pole
from where the path will be opened to expel it. Thicker and stronger fibrosis has
a greater contractile capacity for this expulsion. Moreover, at the pole where the
foreign body will be eliminated, the tissues liquefy in the presence of seroma or
purulent secretion if infected. Again “biological intelligence.”
If it adheres to the fibrous capsule, there may be areas where it remains attached
to the tissues and is exposed where the infection is, for example, polyurethane-coated
implants. Nevertheless, the formation of fibrosis is more intense in all its contours,
always causing capsular contracture.
Furthermore, if this foreign body is not infected and is rigid, it will not change
its shape, and it is surrounded by a layer of fibroblasts/myofibroblasts/fibrocytes/collagen,
which with maturation, try to reduce the volume inside the fibrous capsule, but not
they can and do not deform the rigid body. In the case of breast implants, these layers
of fibroblasts/myofibroblasts/fibrocytes/collagen around them follow the longitudinal
axis of their smooth surface, reducing the volume to be contained within the envelope
formed by them. The more, the greater the contractile force. If this foreign body
is soft, the fibrotic layer that surrounds it will progressively try to reduce its
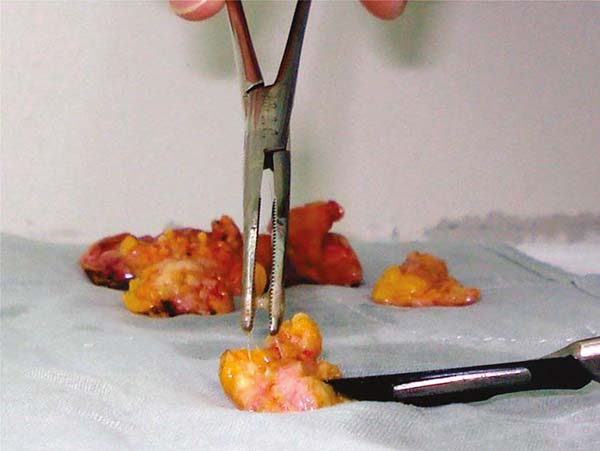
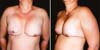
content, and the implant will be bent until, in some cases, it forms a sphere (Figures 7A and 7B).
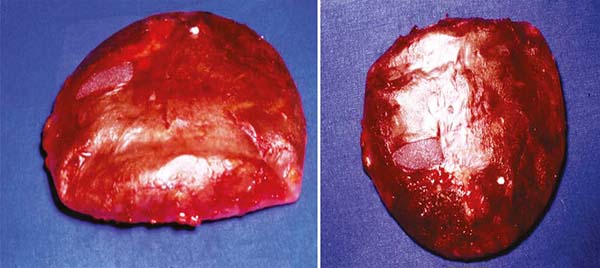
Figure 7 - Implants with capsular contracture removed tending to sphericity.
Figure 7 - Implants with capsular contracture removed tending to sphericity.
The softer the implant, the easier and faster it occurs. The transformation of a three-dimensional
soft foreign body into a sphere occurs because, considering the same volume, the smallest
space occupied by it is the sphere, a convenience with “biological intelligence.”
This explains the rounded shape of sebaceous cysts that become foreign bodies. Thus,
every capsular contracture has a rounded shape, and in a physical inspection of a
breast with a contracted implant, it is rounded and seems to want to “detach” from
the chest (Figures 8).
Figure 8 - Long-term implants with Baker IV capsular contracture with HSD. (Hard, Sore, Deformed).
Figure 8 - Long-term implants with Baker IV capsular contracture with HSD. (Hard, Sore, Deformed).
During the third generation of implants, the number of cases with capsular contractures
increased even more, and in 6 to 12 months, it was rare for a case to have none, even
if of low intensity. Moreover, even supposedly simple tactics to undo them came up.
One of them that was easy to perform was applying a venous pre-anesthetic to the patient,
which breaks the fibrous capsule, pressing the implant with both hands, basically
“squeezing” it. This maneuver was called “external capsulotomy”12,13, or “expansion exercises,” or even “squeeze” (closed capsulotomy by compression)14. This procedure is done in seconds, in a closed system without incisions, increasing
the fibrous continent. Again, misconduct, because it was getting bigger, but in healing,
it almost always contracts again. Sometimes, the excessive manual force also broke
the silicone capsule, which was not cohesive and overflowed to the adjacent broken
tissues. Alternatively, the breast was deformed because the new fibrous capsule did
not regenerate in a defined direction.
According to reports by some Brazilian surgeons of the first and second generations,
the American surgeon who proposed the “squeeze” was because a patient with a contracture
was dating and his partner squeezed his breast very hard, and it softened. After that,
it became a routine among plastic surgeons. In all cases that were made by the author,
it resulted in a new contracture or deformity of the breast. Another basic mistake
was made of not trying to find out why the implants were hard. Breast augmentation
surgery with implants was an adventure and indeed the creation of a problem to be
solved in the future, despite being a simple, quick, and easy to perform the technical
act.
The average time for the events described with the third generation of implants was
around ten years. Hence, the mistaken orientation to exchange them within this period
is sometimes carried out.
Moreover, it was unknown to explain the event when there was unilateral contracture
if biologically it should be all the same in the same patient. It was not taken into
account that the implant is an industrial product subject to industrialization defects;
furthermore, that the executing surgeon could have performed a different maneuver
between the two sides during the surgery, responsible for increasing fibrosis.
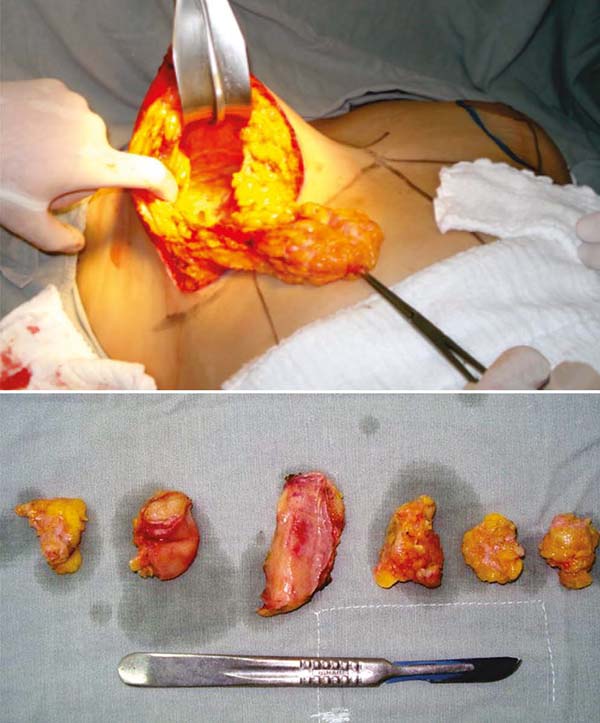
Furthermore, with a longer time, more or less 20 years after implantation, in addition
to contracture, we find fibrous capsules highly contracted and impregnated with calcium
(Figures 9). With such intensity that in some cases, they are similar to an egg, even if the
implant content is saline and leaks (Figure 9). It is deduced then that it is not its content that leads to the foreign body’s
biological reactions over time, but rather, the silicone, of any hardness or cohesiveness
and its capsule. After all, any foreign body that is not contaminated within the tissues
or even own tissues without vascularization becomes “foreign bodies.” The silicone
capsule causes the same foreign body reaction effects even without the inner gel.
Figure 9 - Implant with a filling of extruded saline solution and contracture plus calcification
of the fibrous capsule, removed after 40 years.
Figure 9 - Implant with a filling of extruded saline solution and contracture plus calcification
of the fibrous capsule, removed after 40 years.
The American moratorium that suspended the use of silicone gel by allowing those filled
with saline in 1990/1992 was somewhat misleading, but warned implant manufacturers
to take other steps concerning capsular contracture and gel leaks.
Capsular contracture was the most common and closest late complication in time, and
someone somewhere decided to call it “rejection” this was the most general term among
patients and plastic surgeons, and it has persisted until now, tending to disappear.
The contracture happened in the short, medium, or long term, but it always happened.
In presentations from Brazilian congresses, there were even proposals to include several
small round smooth implants that added together would give the necessary volume, following
the assumption that the fibrous capsule would be less intense and thus avoiding contracture,
but nothing was achieved with this tactic.
Capsular contractures were the reason for Baker, in 19757, to propose graduation of them that would determine the moment of possible implant
replacement. In addition to Baker IV contracture, the author proposed other signs
in the diagnosis of severe contractures, the implants become hard, with changes in
the shape of the breasts (deformed), often with painful symptoms, which was called
the DDD triad (hard, sore) and deformed)15. As early as 1967, a proposal was made to introduce implants into a retromuscular
pocket(16,17 )to give them more excellent protection and prevent capsular contracture; what has
been shown over time is not valid. However, it was the main reason why another pocket
plan was sought. Moreover, since, at that time in Brazil, small implants (120 to 140ml)
were preferred, these were entirely under the larger pectoral muscles. In addition
to breaking in the same way, because the cause was not the inclusion plan, the gel
walked to the armpit more quickly, impregnating tissues and lymph nodes. Furthermore,
the constant muscular contraction caused the implant to rise and flatten, and the
breast to fall, creating a double volume, one on top of the implant and one below
the breast (Figures 10A and 10B). This complication can still exist today.
Figure 10 - Long-lasting sub-muscular implants that have slid upwards over the long term.
Figure 10 - Long-lasting sub-muscular implants that have slid upwards over the long term.
Then the need arose to release the lower insertions of the large pectoral muscles
not to happen. Moreover, over time, the volume of implants requested by the patients
increased, and now when placed in the submuscular plane, they are partially out of
the muscle in the inferolateral region, and during the time and constant contractions,
it pushes it down and to the side, in the direction of ptosis of large and flabby
breasts. Several tactics have emerged from this to allocate them properly without
delaying position changes.
The contracture was inexorable in those implants with the smooth capsule with a smooth
surface, non-cohesive gel, and partial filling, easily seen when placed on a flat
surface, showing irregularities and folding of the envelope in the upper projection
(Figure 2).
Furthermore, even before the American moratorium, the industry realized that the way
to resolve the contracture was the reverse of that initial one. The gel would have
to be cohesive to prevent leakage and harder to avoid contracture. Then came a new
and fourth generation of breast implants. Moreover, almost at the same time, coincidentally,
the coating of the implant started with polyurethane foam (Brazil, 1989), and texturing
of the capsule (Brazil, 1990) still with thin and half-empty capsules. Some companies
already offer the fifth generation with implants with more resistant, fuller capsules,
even harder, more cohesive, and more elastic (more centistokes) gel, in an attempt
to reduce the possibility of contractures. This being a correct path, not abolishing
the contracture, as we cannot forget that it is a soft foreign body subject to the
biological reactions described.
The forces that make this object turn into a round, as mentioned, come from fibroblasts/myofibroblasts/fibrocytes/collagen,
these during maturation will be shorter, involving the implant. They result from these
forces of the whole set of fibrotic capsules directed towards its center, called centripetal
forces (Figures 11A and 11B). Moreover, therefore, when a patient presents herself to the surgeon with
Baker IV and DDD capsular contracture, the breast seems to detach from the chest;
it is round, even more so when there is sagging of the skin, marking its characteristic
contour (Figures 8A and 8B).
Figure 11 - The action of the fibrous capsule centripetal forces on the implant by bending it
in an attempt to make it round towards the center of the smooth implant.
Figure 11 - The action of the fibrous capsule centripetal forces on the implant by bending it
in an attempt to make it round towards the center of the smooth implant.
Many surgical, post-surgical, industrial, and personal occurrences of the patient
herself can increase these centripetal forces. All of them are responsible for the
deposition increase of fibroblasts/myofibroblasts/fibrocytes/collagen in the periphery
of the implant and, consequently, for the increase in the fibrous thickness layer
and the centripetal forces. Are they:
1. Bruises, even if drained, because they impregnate adjacent tissues with blood and
edema; and when absorbed, they cause a greater inflammatory reaction, an increase
in the healing reaction with consequent fibrosis, and more significant contracture.
2. Undetected micro bruises, for the same causes. Single seromas and repeated seromas
aspirated, also for the same causes.
3. Excessive coagulation creating dead tissues that, when eliminated through an inflammatory
reaction, increases fibrosis.
4. Excessive tissue manipulation with severe edema. Gloves contaminated with talc and
clotted blood. Remove and place the implant countless times, and do not take care
of the skin’s sterilization near the incision to introduce the implant.
5. Use non-absorbable products to introduce them, for example, petroleum jelly or tissue
irritants in contact with the implant and the pocket where it will be included.
6. Due to lack of care during the surgery, subclinical infection, mainly by skin germs,
causes recurrent seromas and increased fibrosis. Sometimes requiring the removal of
the implant in a short time, the most common being Staphylococcus aureus.
7. Radiotherapy with radiodermatitis causing skin and subcutaneous tissue stiffness due
to chronic inflammatory reaction. Common in breast reconstructions after radiotherapy,
with an incidence of up to 60%18.
8. Tissue expansion followed by implant insertion. The memory of expanded fabrics tends
to return them to their origin.
9. Mondor’s syndrome, because of the pericapsular lymphatic and venous inflammatory reaction.
It is noted that many of these causes are then linked to the operative technique.
In contrast, the resistance forces that must neutralize the centripetal forces are
the centrifugal forces arising from the implant; their intensity should always be
equal to or more intense than the centripetal forces. They are composed of the gel’s
hardness, the complete filling of the implant, the quality of the silicone capsule,
and its surface characteristics, depending on the implants’ industrialization.
The harder the gel, the greater the centrifugal force of resistance to the contractile
centripetal forces. The more filled the implant, the greater the force. The cleaner
and technically perfect the surgery, the lower the capsular fibrosis and the lower
these forces.
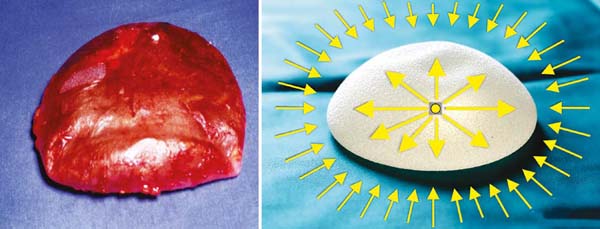
Moreover, the gel’s coating (implant capsule) can reduce or minimize the action of
these centripetal forces of the healing reaction. Therefore, textured implants and
those coated with polyurethane foam appeared. The biological concept is that the smooth
capsule directs the formation of the fibrous envelope, placing the collagen fibers
in the same plane, adding all the centripetal forces resulting from their contraction
towards the implant center. Furthermore, if these directions are varied on the surface,
a good part of them will dissipate (Figure 12). This was the proposal for texturing, as well as covering the implant surface with
polyurethane foam. Nevertheless, the fibrous capsule that is made on the textured
implant should have the “negative” or “mirror” on its surface, male/female type. Moreover,
suppose they are removed for some reason. What is noticeable is a smooth capsular
fibrous surface instead of a rough one, which is always with some content, even if
minimal, of viscous liquid around the implant, similar to synovial fluid, which exists
in the joints, to slip between the rigid articular surfaces, being similar even in
color.
Figure 12 - Centrifugal forces to neutralize centripetal forces dissipated on the implant surface,
depending on hardness, filling, and surface.
Figure 12 - Centrifugal forces to neutralize centripetal forces dissipated on the implant surface,
depending on hardness, filling, and surface.
Could it be that this liquid between the fibrous capsule and the implant produced
to try to prevent constant friction between the two parts, this friction that could
be responsible for late seromas? What about anaplastic large cell lymphoma associated
with breast implantation (BIA-ALCL)?. Well, the future will respond.
Furthermore, implants coated with polyurethane foam are attached to the fibrous capsule
if kept at rest for 60 days, without massage or attitudes that cause the fibrous capsule
to be stray; otherwise, they behave like the textured ones. They often dissolve, producing
a milky white liquid similar to the pus of Staphylococcus aureus infection, but without
a characteristic odor and negative culture, polyurethane tends to be partially absorbed
over time, causing more fibrosis and, therefore, more centripetal force. Could this
lead to less durability for them until reaching the Baker IV contracture with DDD
for replacement, compared to textured surfaces? Despite the almost zero incidences
of capsular contractures in the short and medium-term. Therefore, there is still no
one in the implant market that we could establish as an ideal regarding durability
and shape. So, contracture depends on well-performed surgery and industrialization.
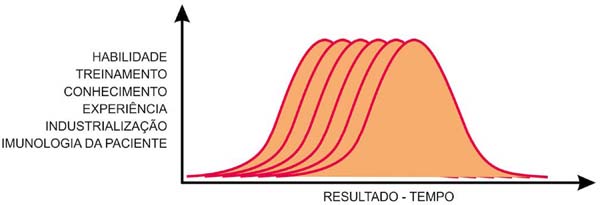
It remains to discuss the immunological capacity of each patient. They are individual,
as are the different biological reactions, not in type, but in intensity and time.
Moreover, this determines why an implant with the same characteristics and the same
industrialization, with a technical act by the same surgeon, has different durability
for different patients (Figure 13).
Figure 13 - Gauss curves, individual and variable for each surgeon (skill, training, knowledge,
experience), each implant industry, and each patient’s immunology.
Figure 13 - Gauss curves, individual and variable for each surgeon (skill, training, knowledge,
experience), each implant industry, and each patient’s immunology.
This immunological area is still poorly studied and poorly described, except that
care should be taken not to use implants in patients with autoimmune disease. However,
there and in the causes mentioned above, it may be the path of industry research to
obtain implants with greater durability. Should we plastic surgeons and implant industries
leave the “comfort zone” (Biggs) and intensify research towards increasing this durability.
With these three factors influencing an implant’s durability, we can say that the
duration of time follows a Gauss curve for each patient, each surgeon, and each industry
(Figure 13).
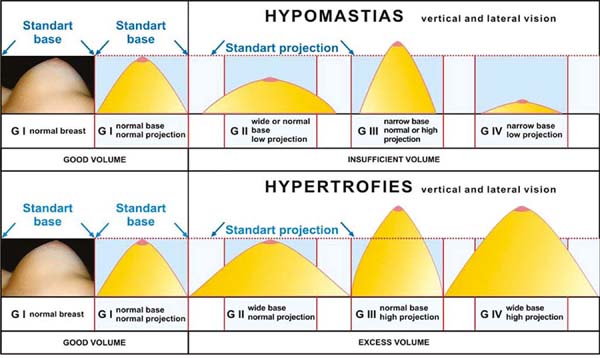
As for the breasts’ anatomical beauty, we must consider that there are several breast
forms of hypomastias, basically four, according to the correlation between the base
and projection diameter. All other variants between these two parameters are derived
from them19 and the total presence or absence of the upper pole. All of this must be added to
the implants’ characteristics (shape/volume and flaccidity), giving the surgeon numerous
options to diagnose and treat hypomastias (Figure 14). And still with some anatomical differences of the chest and its varied dimensions.
In addition to chest and/or muscular changes, congenital or acquired, such as Polland’s
syndrome, pectum scavatum, pectum carinatum, different curvatures of the costal framework, radical mastectomies; we can say that
there is still a lot to research in the development of the ideal forms of breast implants.
Figure 14 - Classification of breasts with hypomastias and hypertrophies by volume and shape,
projection, and extension (diameter) of the base.
Figure 14 - Classification of breasts with hypomastias and hypertrophies by volume and shape,
projection, and extension (diameter) of the base.
It is “art with mathematics” (Biggs), science and technique, and it is much more important
not only to think about volume but also about ideal shapes and proportions. This is
real plastic surgery!
What could that future be in the fifth and sixth generations? Performing a retrospective
analysis of everything that happened, and what is happening, in the first place, there
was not, there is not, and there will be no silicone breast implant with infinite
durability unless the biological reaction of a foreign body is altered. Please pay
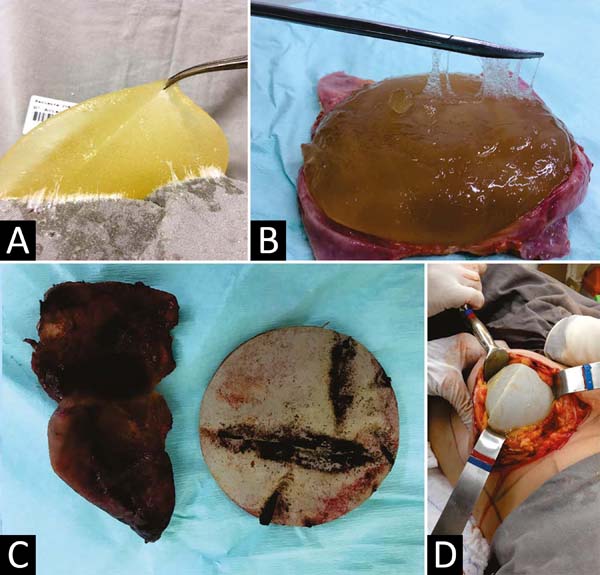
attention because we still find cases of leakage of the gel (Figure 15A) and impregnation of adjacent tissues, dissolution of the capsule (Figure 15B), organized bruises (Figure 15C), or late seromas (Figure 15D), using current implants.
Figure 15 - A: Exudation of cohesive gel impregnating the surgical field. B. Dissolution of the capsule in a recent cohesive gel implant. C. Capsular contracture of a polyurethane-coated implant with chronic intra-capsular
hematoma. D. Seroma and inversion of the polyurethane-coated implant position that did not adhere
to the fibrous capsule.
Figure 15 - A: Exudation of cohesive gel impregnating the surgical field. B. Dissolution of the capsule in a recent cohesive gel implant. C. Capsular contracture of a polyurethane-coated implant with chronic intra-capsular
hematoma. D. Seroma and inversion of the polyurethane-coated implant position that did not adhere
to the fibrous capsule.
Furthermore, the durability is also not ten years, as recommended by some companies
and surgeons. It obeys the Gauss curve of each surgeon, each patient, and each industry.
Moreover, one must also consider each implant itself due to individual defects or
difficulties related to each implantation side.
The closest to the ideal would be an implant with durability between 25 and 30 years
so that there would be only a maximum of two replacements on average throughout life.
Until the use of stem cells and fatty tissue grafts is thoroughly mastered, the implants
will reduce use. It is impossible to obtain implants with high durability and more
varied forms, which would facilitate surgery compared to the use of fat grafting or
stem cells.
Moreover, the fifth or sixth generation could have the implants with the silicone
capsule more resistant, thicker, and more filled, the surface should return to smooth,
avoiding the most intense friction, the gel should be harder and more cohesive, close
to the implant’s glutes, without prejudice to the palpation of a normal breast. Furthermore,
the shapes should have more variations to add to the existing breasts, obtaining perfect
shapes.
Besides, pay attention to rare changes and define other forms of implants rarer, but
made in series or not, including these patients (Polland Syndrome, pectum scavatum, pectus carinatum, etc.). Even if this is an investment with no financial return to industries, they
will certainly be rewarded in other commercial ways. The ultimate medical goal is
the patient’s physical and psychological healing, and the medical device industry
must accompany us.
One should study the biological mechanism by which calcium deposits in the capsules
of foreign bodies and, if possible, postpone this mechanism with some “repellent”
of calcium added to the silicone envelope of the implant in the long term.
Finally, just as the path of industrialization of implants led the industries to manufacture
softer implants because they were hard in the third generation (1980/90), the coincidence
between the external coating and the increase in the hardness and cohesiveness of
the gel led to think that it was the coating that reduced the incidence of capsular
contractures. Nevertheless, we must think and analyze that the responsible for this
may have been the hardness, the gel’s cohesiveness, the complete filling of the capsule,
and not the implant surface’s characteristic.
REFERENCES
1. Cronin TD, Gerow FJ. Augmentation mammaplasty: a new "natural prothesis". In: Third
International Congress of Plastic and Reconstructive Surgery, Washington, 1963. Excerpta
Medica, Amsterdam; 1963. p. 41-9.
2. Peterson HD, Burt G. The role of steroides in the prevention of circunferential scarring
in augmentation mammaplasty. Plast Reconstr Surg. 1974;54:28-30.
3. Perrin ER. The use of soluble steroids with inflatable breast prosthesis. Plast Reconstr
Surg. 1976;57:163.
4. Imber G, Schwager RG, Guthrie Junior RH, Gray GF. Fibrous capsule formation after
subcutaneus implantation of synthetic materials in experimental animals. Plast Reconstr
Surg. 1974;54:183-6.
5. Wilflingsseder P, Propst A, Mikuz G. Constructive fibrosis following silicone implants
in mammary augmentation. Chir Plast. 1974;2:215-17.
6. Williams C, Aston S, Rees TD. The effect of hematoma on the thickness of pseudosheaths
around silicone implants. Plast Reconstr Surg. 1975;56(2):194-8.
7. Baker J. Classification of spherical contractures. In: Aesthetic Breast Symposium.
Scottsdale, Arizona; 1975.
8. Kumar V, Abbas AK, Fausto N, Aster JC, orgs. Robbins e Cotran Patologia - bases patológicas
das doenças. 8ª ed. New York: Saunders Elsevier; 2004.
9. Andrews JM. Biomateriais em Cirurgia Plástica. In: Mélega JM, Zanini AS, Psillakis
JM, eds. Cirurgia plástica reparadora e estética. 3ª ed. Rio de Janeiro: Medsi; 1988.
p. 111-8.
10. Sperli A, Bersou Junior A, Freitas JOG, Michalany N. Complicações com próteses mamárias.
Rev Bras Cir Plást. 2000;15(3):33-46.
11. Baker Junior JL, Chandler ML, LeVier RR. Ocurrence and activity of myofibroblasts
in human capsular tissue surrouding mammary implants. Plast Reconstr Surg. 1981 Dez;68(6):905-12.
12. Baker JL, Bartels RJ, Douglas WM. Closed compression technique for rupturing a contracted
capsule around a breast implant. Plast Reconstr Surg. 1976;58:137.
13. Vinnik CA. Spherical contracture of fibrous capsules around breast implants. Plast
Reconstr Surg. 1976 Nov;58(5):555-60.
14. Little G, Baker Junior JL. Results of closed compression capsulotomy for treatment
of contracted breast implants capsules. Plast Reconstr Surg. 1980;65:30-3.
15. Bozola AR, Bozola AC, Carrazzoni RM. Inclusão de próteses mamarias de silicone-poliuretano.
Rev Soc Bras Cir Plást. 2006;21(1):18-22.
16. Sanvenero-Rosselli G. The submuscular implant in augmentation mammaplasty. In: Transactions
of the Fourth International Congrees of Plastic and Reconstructive Surgery, Rome,
October 1967. Quadrennial Meeting of the Plastic and Reconstructive Surgery.
17. Griffiths CO. The submuscular implant in augmentation mammaplasty. In: Translations
of the Fourth International Congress of Plastic Surgery. Amsterdam: Excerpta Medica
Foundation; 1967. p. 1009.
18. Forman DL, Chiu J, Restifo RJ, Ward BA, Haffty B, Ariyan S. Breast reconstruction
in previous irradiated patients using tissue expanders and implants: a potentially
unfavorable result. Ann Plast Surg. 1998 Abr;40(4):360-3.
19. Bozola AR, Longato FM, Bozola AP. Análise geométrica da forma da beleza e da forma
de prótese baseado na proporção Phi: aplicação prática. Rev Bras Cir Plást. 2011;26(1):94-103.
1. Faculty of Medicine, São José do Rio Preto, SP, Brazil.
Corresponding author: Antonio Roberto Bozola, Avenida Brigadeiro Faria Lima, 5544, Vila São José, São José do Rio Preto, SP, Brazil.
Zip Code: 15090-000. E-mail: ceplastica@hotmail.com
Article received: September 23, 2019.
Article accepted: July 15, 2020.
Conflicts of interest: none