INTRODUCTION
Postoperative Cullen gangrene, also called pyoderma gangrenosum (PG)1 or sterile neutrophilic abscess (because lesions do not contain pathogenic micro-organisms)
was first described in the medical literature by Cullen in 19241. Brusting et al.
then described PG in greater detail in 19302.
The etiology of PG is unknown. In most cases, PG is related to malignant neoplasms,
rheumatologic diseases, arthritis, inflammatory bowel diseases such as ulcerative
colitis or Crohn’s disease, monoclonal gammopathies, collagenosis, Behcet’s disease,
Wegener’s granulomatosis, myeloproliferative diseases, and infectious diseases, especially
hepatitis and Acquired Immunodeficiency Syndrome (AIDS)2.
PG is characterized by painful ulcers of various sizes and depths with ill-defined
borders. PG has no neoplastic origin, nor is it associated with primary vasculitis.
Though PG lesions may suffer from secondary infection, the underlying pathology of
PG is unrelated to infection3.
Pyoderma gangrenosum is a rare inflammatory neutrophilic dermatosis occurring in 3
to 10 cases per million per year. In most cases, PG is chronic and relapsing. The
condition commonly affects adults between 20 and 50 years old, and is more common
in women than men. Children and adolescents constitute only 4% of cases1-5. One retrospective study found that the incidence of PG in Brazil was 0.38 cases
per 10,000 hospital visits6.
In this report we detail a rare case of pyoderma gangrenosum caused by blunt trauma
to the dorsum of the hand. The patient was treated in the Plastic Surgery Unit of
t he Regional Hospital of Asa Norte (HRAN), Brasília-DF.
CASE REPORT
A 33-year-old male Caucasian patient was the victim of a work-related accident with
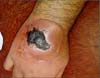
a sledgehammer, resulting in trauma to the dorsum of the right hand (Figure 1). The patient was a mechanic and did not report having any comorbid conditions.
Figure 1 - Traumatized region with ecchymosis.
Figure 1 - Traumatized region with ecchymosis.
Edema appeared immediately after the trauma, which developed into a large hematoma
over the next few days. The hematoma was surgically drained at the patient’s local
health clinic. After the drainage, a chronic wound formed on the dorsum of the patient’s
hand (Figure 1).
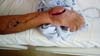
The patient was referred to a specialist in Goiânia, and was subjected to a Chinese
flap procedure (Figure 2). Ten days after the surgery, the edge of the flap detached from the wound bed. Necrosis
at the wound edges soon spread, resulting in the total loss of the flap (Figure 2). After debridement and wound preparation, a biopsy of the exudative found signs
of chronic inflammation and negative cultures.
Figure 2 - Chinese flap with necrosis.
Figure 2 - Chinese flap with necrosis.
A new flap procedure was performed 36 days after the loss of the first. The new posterior
interosseous fasciocutaneous flap underwent the same evolution as the first. The edge
of the flap detached from the wound bed, after which necrosis spread outwards from
the wound’s edges. Although without any early suffering in the two vascular procedures,
a new wound reopened (Figures 3 and 4).
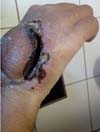
Figure 3 - Peripheral detachment of the posterior interosseous flap from the wound bed.
Figure 3 - Peripheral detachment of the posterior interosseous flap from the wound bed.
Figure 4 - Distal necrosis of the posterior interosseous flap.
Figure 4 - Distal necrosis of the posterior interosseous flap.
The patient was then referred to our Plastic Surgery Unit at the HRAN in Brasilia.
A superficial debridement of the necrotic tissue was performed, and new tissue and
bone samples were collected for culture and biopsy. The resulting histopathological
tests were inconclusive. An AFB test was negative, microscopy for leishmania was negative,
and cultures for fungus, tuberculosis, and aerobic bacteria were negative. The patient
only displayed signs of acute chronic inflammation without signs of neoplasia.
After clinical discussion within the unit and exclusion of other diagnoses, we suspected
pyoderma gangrenosum. No further surgical debridement was performed in the HRAN. The
patient began systemic corticosteroid therapy (maximum dose of prednisone: 70 mg 1x/day)
and triamcinolone was applied to the edge of the wound. The patient’s condition improved,
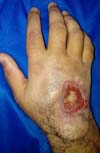
and the wound almost spontaneously closed after showing signs of granulation (Figure 5). A few months after the patient was discharged, he once again experienced necrosis
resulting in joint pain and bone exposure. Administration of morphine in the patient’s
home city resulted in only partial analgesia.
Figure 5 - Almost total wound closure after systemic corticosteroids and local infiltration.
Figure 5 - Almost total wound closure after systemic corticosteroids and local infiltration.
The patient was treated by his local rheumatologist and orthopedist, who treated him
with corticosteroid therapy. The patient then suffered from a bone infection. Complementary
radiological exams were performed. The patient showed signs of acute osteomyelitis
with onset of sepsis, and reported that his joint pain did not respond to intravenous
morphine. Physicians therefore opted to amputate the patient’s hand at the wrist (Figure 6).
Figure 6 - Amputation of the hand.
Figure 6 - Amputation of the hand.
The patient symptoms significantly improved after surgery, and the patient did not
experience further pain. However, the patient showed signs of pathergy in his lower
limbs after a mild local trauma to his ankle. The trauma triggered the opening of
new wounds in the dorsum of the foot and in the anterior portion of the thigh. The
patient was treated with the immunomodulator Adalimumab along with Methotrexate, after
which the patient’s wounds improved (Figures 7 and 8).
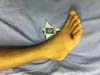
Figure 7 - Pathergy on the dorsal aspect of the foot after mild trauma.
Figure 7 - Pathergy on the dorsal aspect of the foot after mild trauma.
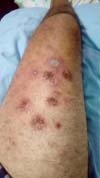
Figure 8 - Pathergy in the thigh after trauma to the foot (pustules).
Figure 8 - Pathergy in the thigh after trauma to the foot (pustules).
DISCUSSION
Because a histopathological exam is not sufficient evidence to diagnose PG, a PG diagnosis
is based on clinical evidence. Edema, signs of massive inflammation caused by neutrophils,
engorgement, thrombosis of small and medium vessels, necrosis, and hemorrhage are
often seen in PG patients. The formation of abscesses and necrosis results in tissue
liquefaction and secondary thrombosis of venules, allowing PMN leukocytes to infiltrate
the region. The lesions originate from suppurative granulomatous dermatitis, and regress
with remarkable fibroplasia6.
There is evidence to suggest that PG is an autoimmune disease, specifically caused
by immune-induced cellular autophagy. This explanation is consistent with this case,
and would help explain the loss of the two flaps constructed to cover the patient’s
initial wound6,7.
The immunological reaction involves capillary vasodilation, causing a cutaneous rash.
This process initiates a sequence of reactions, resulting in intense neutrophil migration
to the region. The neutrophils remain in the basal layer of the skin and produce large
amounts of collagenase, which degrades both the collagen and the capillaries of the
basal layer. The bonds holding the basal layer together then degrade, opening the
skin and causing necrosis. Necrosis, in turn, attracts even more neutrophils and macrophages
to the site. These neutrophils produce more collagenase that destroys more cutaneous
tissue, resulting in a positive feedback cycle6,7.
The clinical presentations of PG are variable, and include the rare bullous, pustular,
and vegetative forms of PG. Even more uncommon forms include PG at sites of pathergy
(in 20-30% of cases), the periostomal skin, the dorsum of the hand, and the head and
neck. PG can also be multisystemic and paraneoplastic6,7.
PG usually begins as a deep and painful nodule or a superficial hemorrhagic pustule,
sometimes resulting from minor trauma. A painful and ulcerated lesion with irregular
borders then forms. These lesions have an inflammatory and elevated aspect, appear
dark reddish or purple, and have a granular necrotic background with slight abscesses6,7.
In their ulcerated form, the interiors of PG lesions produce a hemorrhagic, purulent
exudate. These lesions spread in a serpiginous pattern through the excavation of lesion
edges or the appearance of new hemorrhagic pustules.
When superficial, PG lesions can be confined to the dermis. However, more commonly
PG lesions expand into subcutaneous tissue and fascia, ultimately exposing muscle
and bone.
Multiple PG lesions can appear gradually and simultaneously in different locations,
especially in the lower limbs, abdomen and buttocks. Interestingly, the mucous membranes
are usually spared. However, aphthous lesions may occur in the oral cavity, sometimes
massively involving the pharynx and larynx.
PG can progress in two main patterns: (1) an explosive beginning involving the rapid
spread of lesions, pain, fever, systemic toxicity, hemorrhagic phlyctenae, suppurations,
and an inflamed halo around wound edges; or (2) a slow progression involving massive
granulations inside ulcers, crust and
hyperkeratosis at wound edges, and the spontaneous regression of some lesions while
other lesions progress eventually spreading to extensive areas8,9.
The four distinct clinical and histopathological forms of PG are:
Propagation - corresponding to 12.5% of cases10: Also called superficial granulomatous pyoderma, this is the most localized and least aggressive form of PG. Patients present with
superficial lesions that have verrucous aspects and non-purulent backgrounds. Lesions
occur on the torso, head and neck. Rapidly responds to appropriate therapy.
Bullous - 6.25% of cases10: lesions have an acute onset and are associated with leukemic frameworks. Lesions
are superficial and involve papules, purpura and bluish bullae, and hemorrhaging.
Ulcerative - 81.52% of cases10: lesions begin as small pustules surrounded by an inflammatory halo. Lesions are
painful and evolve rapidly. As lesions resolve, an atrophic scar and an epidermis
with a “cigarette paper-like” aspect are visible11.
Pustular - a rare presentation associated with fever, arthralgia, and intestinal
inflammatory diseases. Lesions occur mainly in the extensor surface of the extremities.
After the intestinal pathology is controlled, the disease may regress without leaving
scars, although the lesions may coexist with the ulcerative form10,11.
PG is diagnosed clinically, mostly by excluding similar presentations. Cultures for
fungi and bacteria are generally negative, and the results of histopathological examination
are compatible with neutrophilic dermatosis6-11. These general clinical features were consistent with this case, and the results
of multiple biopsies and negative cultures are consistent with a diagnosis of ulcerative
and pustular PG (Figure 8).
PG may appear after trauma or surgery, and is often confused with the infection of
a surgical wound. Proper treatment for PG is performed systemically, and importantly
surgical debridement is not recommended due to its potential to promote pathergy12,13.
To illustrate this principle, the two surgical procedures performed in this case resulted
in flap loss, and local debridement reactivated and worsened the patient’s necrosis.
In 50 to 70% of patients, PG is associated with an underlying disease such as inflammatory
bowel disease, rheumatic diseases, hematological diseases or malignancy, hepatitis
B and C, AIDS, systemic lupus erythematosus, psoriasis, or reactive arthropathies3,14,15.
In this case, there was no other pathology associated with PG.
Fever, malaise and myalgias have been reported in some PG patients. Variable presentations
of arthritis are present in 37% of cases, which include classic arthritis, asymmetric
arthritis in the lower limbs, and monoarthritis3. In this case, the patient presented with high intensity arthralgia and fever, and
myalgia that was unresponsive to morphine.
Pathergy can occur in up to 25% of cases, in which new lesions appear as a result
of trauma such as insect bites, intravenous injections, debridement, and even biopsy.
Pathergy can also occur after gynecological surgical procedures as reported by Meyer
et al. in 200616, after partial thickness skin grafts as reported by Coltro et al. in 200617, and after mammoplasty as reported by Soares et al. in 201318.
Pathergy was also observed in this case. Specifically, the patient’s condition deteriorated
after surgical trauma and debridement. Pathergy was also observed after mild trauma
to the foot, which led the emergence of a new lesion next to the trauma site (Figures 7 and 8).
The bullous form of PG is also associated with hematological disorders such as leukemia
and myelodysplastic syndrome (MDS). Indeed, 54% of bullous PG cases are associated
with leukemia18.
Therapeutic alternatives include immunomodulators, corticosteroids, and immunosuppressants.
The patient’s treatment depends on the specific presentation of PG, as well as the
doctor’s experience regarding proper drug selection19.
PG is treated through immunosuppression. Local treatments should only be used for
very small and early lesions. The affected area should be kept clean and moist. Occlusive
dressings or hydrogel can be used.
Surgical debridement should be avoided, since the resulting pathergy can worsen the
patient’s condition.
Intralesional triamcinolone infiltration (20 mg/ mL in monthly applications) can lead
to remission after five to eight weeks3. While such treatment improved our patient’s
condition initially, his condition worsened near the end of triamcinolone treatment
(Figure 5).
Biweekly intralesional injections of cyclosporine (1:3 solution in saline) are another
alternative treatment for PG.
The vast majority of PG patients undergo systemic treatment, while local therapy is
used as adjuvant. High doses of corticosteroids (prednisone or prednisolone orally,
1-3 mg/kg/day) can be administered during the early stages of PG. Corticosteroids
can also be administered in the form of pulse therapy (methylprednisolone 1 g/day
for three days). After the disease has been controlled, the dose of corticosteroids
is gradually reduced. However, in this case the patient did not respond to corticosteroid
therapy20-23.
Cyclosporine is the gold standard treatment for PG. The majority of PG cases respond
well to relatively low doses of cyclosporine (3-6 mg/kg/day). It is important to monitor
blood pressure, renal function, hepatic function, and triglyceride levels during cyclosporine
therapy. Other options include tacrolimus, azathioprine, dapsone, thalidomide, and
clofazimine21.
Recent reports show that Infliximab can successfully treat cases of PG that were resistant
to other therapies21. A combination of methotrexate and adalimumab produced a good clinical response in
our patient.
Because the fundamental therapeutic principle PG treatment is immunosuppression, the
possibility of infection must be completely excluded before commencing treatment.
Surgery has therapeutic value in the final stages of PG. After total remission of
PG, grafts or flaps may be required to close large areas affected by skin loss23.
Pyoderma gangrenosum of the hand is rare and is usually confused with infection. In
a series of seven cases published by Huish et al. in 200124, PG was misdiagnosed 13 times (ranging from 1-3 times per patient) resulting in 16
unnecessary surgeries (an average of 2.2 per patient) including four amputations and
two failed skin grafts. Incorrect diagnoses lead to unnecessary treatments, surgeries,
and even the appearance of sudden-onset PG 24.
It is important to classify PG’s clinical forms, establish associations between PG
and underlying pathologies, and to evaluate the immune response in PG. The many drugs
used to treat PG demonstrate the difficulty of standardizing treatment. Physicians
can use an empirical approach to select the appropriate drug for each patient. The
administration of corticosteroids, immunosuppressants and immunomodulators (combined
with local care) can stop the progression of the disease. There are also rare reports
of resistance to these medications. Such patients have a reserved prognosis, as in
our case, in which worsening PG eventually led to amputation.
REFERENCES
1. Schofer H, Baur S. Successful treatment of postoperative pyoderma gangrenosum with
cyclosporin. J Eur Acad Dermatol Venereol. 2002;16(2):148-51.
2. Brusting LA, Goeckerman WH, O’Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and
experimental observations in 5 cases occurring in adults. Arch Derm Syphilol. 1930;22(4):655-80.
3. Blitz NM, Rudikoff D. Pyoderma gangrenosum. Mt Sinai J Med. 2001;68(4-5):287-97.
4. Serra-Baldrich E, Boixareu MJT. Pioderma gangrenoso: consideraciones. Act Dermatol.
2001;4(2):115-22.
5. Tanus R, Cassol T, D’Aquino Neto V. Pioderma gangrenoso em membro inferior: relato
de caso. Arq Catarin Med. 2009;38(Supl 1):70-2.
6. Graças AM, Alecrim ES, Lyon S. Pioderma gangrenoso: evidências clínicas e características.
Rev Med Minas Gerais. 2016;26:e-1790.
7. Konopka CL, Padulla GA, Ortiz MP, Beck AK, Bitencourt MR, Dalcin DC. Pioderma gangrenoso:
um artigo de revisão. J Vascul Bras. 2013;12(1):25-33.
8. Hadi A, Lebwohl M. Clinical features of pyoderma gangrenosum and current diagnostic
trends. J Am Acad Dermatol. 2011;64(5):950-4.
9. Wani I, Bhat IHG, Mir M, Mir M, Hassan N, Mustafa A. Pyoderma gangrenosum of abdominal
wall: a case report. Oman Med J. 2011;26(1):64-5. DOI: http://doi.org/10.5001/omj.2011.18
10. Conrad C, Trüeb RM. Pyoderma gangrenosum. J Dtsch Dermatol Ges. 2005;3(5):334-42.
DOI: http://dx.doi.org/10.1111/j.1610-0387.2005.05022.x
11. Newman B, Cescon D, Domenchini A, Siminovitch KA. CD2BP1 and CARD 15 mutations are
not associated with pyoderma gangrenosum in patients with inflammatory bowel disease.
J Invest Dermatol. 2004;122(4):1054-5. DOI: http://dx.doi.org/10.1111/j.0022-202X.2004.22430.x
12. Zold E, Nagy A, Devenyi K, Zeher M, Barta Z. Successful use of adalimumab for treating
fistulizing Crohn’s disease with pyoderma gangrenosum: two birds with one stone. World
J Gastroenterol. 2009;15(18):2293-5. DOI: http://dx.doi.org/10.3748/wjg.15.2293
13. Souza CS, Chioss MPV, Takada MH, Foss NT, Roselino AMF. Pioderma gangrenoso: casuística
e revisão de aspectos clínico-laboratoriais e terapêuticos. An Bras Dermatol. 1999;74(5):465-72.
14. Beber AAC, Knob CF, Shons KRR, Neumaier W, Silva JCN, Monticielo OA. Pioderma gangrenoso
associado à artrite reumatoide: descrição de caso. Rev Bras Reumatol. 2014;54(4):322-5.
15. Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective
review of patient characteristics, comorbidities and therapy in 103 patients. Br J
Dermatol. 2011;165(6):1244-50. DOI: https://doi.org/10.1111/j.1365-2133.2011.10565.x
16. Meyer TN. Pioderma gangrenoso: grave e mal conhecida complicação da cicatrização.
Rev Bras Cir Plást. 2006;21(2):120-4.
17. Coltro PS, Valler CS, Almeida PCC, Gomez DS, Ferreira MC. O papel da patergia no pioderma
gangrenoso em áreas doadoras de enxertos cutâneos: relato de caso. Rev Bras Cir Plást.
2006;21(4):231-5.
18. Soares JM, Rinald AE. Pioderma gangrenoso pós-mamoplastia redutora: relato de caso
e discussão. Rev Bras Cir Plást. 2013;28(3):511-4.
19. Batista MD, Fernandes RL, Rocha MAD, Ikino JK, Pinheiro RF, Chauffaille MLF, et al.
Pioderma gangrenoso bolhoso e síndrome mielodisplásica. An Bras Dermatol. 2006;81(5
Supl 3):S309-12.
20. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum:
a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. DOI: http://dx.doi.org/10.2165/11595240-000000000-00000
21. Lazarus GS, Goldsmith LA, Rocklin RE, Pinals RS, De Buisseret JP, David JR, et al.
Pyoderma gangrenosum, altered delayed hypersensitivity and polyarthritis. Arch Dermatol.
1972;105(1):46-51. DOI: http://dx.doi.org/10.1001/archderm.1972.01620040018003
22. Friedman S, Marion JF, Scherl E, Rubin PH, Present DH. Intravenous cyclosporine in
refractory pyoderma gangrenosum complicating inflammatory bowel disease. Inflamm Bowel
Dis. 2001;7(1):1-7. DOI: http://dx.doi.org/10.1097/00054725-200102000-00001
23. Miller J, Yentzer BA, Clark A, Jorizzo JL, Feldman SR. Pyoderma gangrenosum: a review
and update on new therapies. J Am Acad Dermatol. 2010;62(4):646-54. DOI: http://dx.doi/10.1016/j.jaad.2009.05.030
24. Huish SB, Bountiful UT, De La Paz EM, Cincinnati OH, Ellis PRIII, Dallas TX, et al.
Pyoderma gangrenosum of the hand: a case series and review of the literature. J Hand
Surg Am. 2001;26(4):679-85.
1. Hospital Regional da Asa Norte, Brasília, DF, Brazil.
Corresponding author:
Altino Vieira de Rezende Filho Neto, Setor SMAS, Trecho 1, Lote C, Bloco J, Brasília, DF, Brazil. Zip Code: 71218-010.
E-mail: altinofn@hotmail.com
Article received: January 21, 2019.
Article accepted: April 21, 2019.
Conflicts of interest: none.