INTRODUCTION
Keloids are abnormal, benign, erythematous, fibrous proliferations formed on the skin
and usually develop after dermal trauma caused by burns, surgeries, acne, piercings,
and tattoos.1 Healing extends beyond the wound margins surrounding adjacent normal skin2 (Figures 1 and 2).
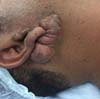
Figure 1 - Ear lobe keloid. Image courtesy of Professor Joyce de Sousa Fiorini Lima.
Figure 1 - Ear lobe keloid. Image courtesy of Professor Joyce de Sousa Fiorini Lima.
Figure 2 - Keloid scar in the pre-sternal region. Image courtesy of Prof. Joyce de Sousa Fiorini
Lima.
Figure 2 - Keloid scar in the pre-sternal region. Image courtesy of Prof. Joyce de Sousa Fiorini
Lima.
The pathogenesis involves excessive fibroblast proliferation and abnormal collagen
production. Wound tension and infectious processes promote hypoxia and increased deposition
of extracellular matrix components leading to the formation of thick collagen bundles
with a hyaline aspect. Lesions usually occur on the face, ear lobes, trunk, and neck
potentially causing functional and cosmetic impairments, which may lower self-esteem
and quality of life1.
The incidence and prevalence of keloids are unknown but affect predominantly young
adults. Moreover, the prevalence varies according to race and is higher in Black and
Asian populations, but similar between sexes. Keloids have a genetic basis, with an
autosomal dominant inheritance of incomplete penetrance and manifest in genetic syndromes3.
The diagnosis is usually clinical and based on medical histories as well as the shape,
size, and growth pattern of lesions. Biopsies may be performed in cases in which diagnoses
are uncertain. The most common symptoms are pain and itching. After burn injuries,
temperature dysregulation, dry skin, neuropathic pain, and impairment of mechanical
function may occur due to destruction of hair follicles, sweat glands, capillaries,
and nerve endings4.
The objective of treatment is to reduce complaints and scar volume and promote functional
and cosmetic improvements. Furthermore, the chosen surgical approach needs to include
the possible postoperative risk of overhealing.
It is known that the delay in epithelialization is related to an increased occurrence
of keloids. Therefore, it is necessary to stimulate rapid re-epithelization with respect
to physiological mechanisms and skin anatomy5.
For this reason, the risk of recurrence is a significant limiting factor in therapeutic
success. Recurrence is more common in body regions subject to mechanical stress, and
increasing patient awareness regarding this fact is fundamental. Current treatment
options fail to achieve satisfactory scar reduction. Moreover, despite the large number
of treatment options, their quality of evidence is low and intervention outcomes have
poor consistency and predictability. This limitation has caused confusion about which
treatments and techniques should be considered primary, secondary, or tertiary.
OBJECTIVE
The objectives of this review were to outline the main treatment options for keloids
and address their advantages and disadvantages.
METHODS
A non-systematic literature review of keloids was conducted using PubMed, Scielo,
DynaMed, MEDLINE, UptoDate, and textbooks in dermatology and dermatological surgery
to identify the main surgical and adjuvant therapies. The descriptors used were keloids,
treatment, surgery, and healing.
LITERATURE REVIEW
Keloids can become lesions that are healed in a timely manner preventing the development
of the pathophysiological features of the original lesion. Current surgical approaches
should include adjuvant therapies to avoid the risk of recurrence potentially leading
to the formation of larger keloids. The most commonly used therapies are:
3.1 - Excision
Excision consists of the surgical removal of the keloid and is considered a secondary
or tertiary treatment option for mature keloids6. The fusiform excision of lesions at an angle of 30° to skin tension lines is recommended.
Recurrence can be avoided by reducing the tension at wound closure, leaving the edges
everted, and using resorbable thread in the fascia or subcutaneous tissue7.
Avoiding excessive manipulation and trauma, removing foreign bodies, and preventing
bruises and infections help improve the quality of scars. Surgical correction by Z-plasty
or W-plasty is possible8.
Surgical excision alone should be avoided because of the risk of recurrence in 45-100%
of cases, and therefore should be associated with adjuvant therapies. The use of pressure
dressings after surgery is also recommended9.
Surgical excision combined with radiotherapy is an effective treatment for large keloids
according to international clinical recommendations; however, this approach should
be used as a last resort. The combination of surgery with radiotherapy in patients
who failed a first treatment increased the recurrence rate from 8% to 28%10. A previous study demonstrated that surgical excision of foot keloids associated
with intralesional corticosteroid injections and silicone gel sheeting decreased the
1-year recurrence rate by 78.5%11.
3.2 - Intralesional injections
Intralesional injections involve the intralesional application of medications such
as corticosteroids. The most commonly used medication is triamcinolone acetonide which
is considered a primary therapy and is usually combined with silicone gel sheeting
for keloid scars smaller than 0.5 cm. It is also the first-choice treatment in isolation
or combination with intralesional fluorouracil or cryotherapy for keloids larger than
0.5 cm6.
Corticosteroids act by decreasing the synthesis of collagen and glycosaminoglycan
and inhibiting fibroblast proliferation. These drugs are effective in reducing pain
and itching because of their anti-inflammatory and vasoconstrictive properties. The
rate of response to treatment is 50-100%, and the 5-year recurrence rate is ≤50%.
Injections are painful and have adverse effects in 63% of cases, including dermal
atrophy, hypopigmentation, and telangectasis7.
Cryotherapy facilitates corticosteroid injection but causes residual vitiligo. The
use of thermal bags before cryotherapy applications and slow administration can reduce
pain1. Injectable corticosteroids are also used in adjuvant therapy after surgical excision7, whereas the use of triamcinolone after surgical excision is effective for keloids
larger than 4 cm12.
Intralesional verapamil may be as effective as intralesional triamcinolone in treating
keloids but has fewer adverse effects13. However, a randomized study found that the risk of recurrence using verapamil was
higher14. Another randomized trial demonstrated that the effectiveness of the two treatments
was similar, but the delay in treatment outcomes was longer with verapamil15.
Intralesional 5-fluorouracil (5-FU) is used for lesions refractory to corticosteroid
treatment. This antimetabolite chemotherapeutic agent interferes with DNA and RNA
synthesis and induces fibroblastic apoptosis; it also inhibits type I collagen production
by affecting transforming growth factor-beta (TGF-β) signaling16.
The adverse effects of 5-FU include pain and hyperpigmentation7. This medication can be used together with intralesional corticosteroids and as an
adjuvant after surgical excision8. The rate of success of treatment in isolation and in combination with corticosteroids
is 45-78% and 50-96%, respectively. However, the quality of evidence of 5-FU treatments
is low17.
Botulinum toxin type A injections have been used as a new treatment option. It acts
by decreasing tension in the tissue near the keloid scar during the healing process
and affecting apoptosis and cell proliferation. A study found that the rate of recurrence
in 80 patients with keloid lesions who underwent excisional surgery followed by a
single application of 5-FU and botulinum toxin on day 9 after surgery was 4%. Therefore,
the authors recommended the routine application of this toxin after resection; however,
further studies are needed to assess the effectiveness of this approach18.
3.3 - Dermal regeneration matrix
The dermal regeneration matrix (DRM) (Integra®) consists of a bilaminar structure
formed by a dermal component (bovine collagen and chondroitin-6-phosphate) and an
epidermal component made with a 100 µm thick synthetic silicone sheet19.
It has a three-dimensional structure with 50 ± 20 µm pores and resembles normal skin.
The matrix is completely degraded after 30 days. The outer silicone layer acts as
a protective barrier and prevents the loss of liquids. After neovascularization, the
silicone sheet is replaced with a thin autologous epidermal graft, and the epidermal
cells in the grafts proliferate and attach to the neodermis19.
DRMs promote wound healing without wound tension, do not trigger immune responses,
contain a thin epidermal graft, do not require adjuvant therapies, and the results
resemble healthy skin. DRMs are sterile and their implantation is relatively simple.
The disadvantages are its high cost, risk of infection between the two layers of the
matrix, and the need for two surgical procedures19,20.
A study evaluated the use of DRMs for keloids and found that the aesthetic results
were acceptable and that the skin remained flat and infection free. Moreover, there
were no long-term complaints or evidence of scarring at donor sites. Limitations included
a small sample size and a follow-up period of <24 months20.
3.4 - Cryotherapy
Cryotherapy involves freezing keloids with liquid nitrogen. Studies recommend the
monthly administration of intralesional corticosteroids with or without adjuvant cryotherapy
as the primary treatment for large keloids6.
The mechanism of action involves cellular and microvascular damage, leading to necrosis
and consequent involution of the lesions without affecting the connective tissue framework.
This approach induces immune modulation and tumor cell apoptosis. The mechanism of
action depends on the freezing and thawing rate, tissue type, and temperature9.
This therapy causes rapid freezing of the scar tissue from the lesion center to the
outer superficial area. Cryotherapy differs from cryogenic contact therapy, which
partially freezes the lesion. Intralesional cryotherapy can be performed using a probe,
which is inserted in the scar and allows dermal targeting without affecting the epidermis21.
Side effects include blisters, pain, and hypo- or hyper-pigmentation. A study reported
a 67.4% reduction in keloid volume in a 6-month period using a single session of intralesional
cryotherapy, as well as improvement in redness and absence of hypopigmentation and
recurrence9.
Evidence suggests that intralesional cryotherapy reduces scar volume and symptoms
and has few adverse effects. However, more unbiased, robust, and comparative studies
are needed to confirm this evidence21. A randomized trial demonstrated that intralesional cryotherapy and brachytherapy
were less effective than surgical excision in patients with resistant keloids. Intralesional
cryotherapy also reduces the volume of primary keloids22.
3.5 - Laser therapy
Targeted treatments include pulsed dye laser (PDL), carbon dioxide ablative fractional
resurfacing (AFR), and intense pulsed light (IPL)23. PDL or AFR are indicated for smaller keloids refractory to silicone gel sheeting,
intralesional corticosteroids, and 5-FU24.
Combined treatments, such as PDL and AFR, are recommended over single treatments by
improving the aesthetic results and reducing symptoms25.
The most common complications of PDLs are transient purpura, mild or moderate erythema,
and edema. Blistering or scabbing of the skin may occur in the early post-treatment
stages. Hypo- or hyper-pigmentation may also occur, especially in patients with darker
skin. AFRs may cause delayed healing, ulcers, and post-inflammatory hyperpigmentation23,26.
3.6 - Silicone gel sheeting
Silicone gel sheeting is used as a primary therapy to prevent and treat keloid scars
smaller than 0.5 cm in moderate to high-risk patients6,26. The anti-scarring mechanism is unclear, but may be related to occlusion and hydration
of the stratum corneum, generation of static electricity, and decreases in the number
of mast cells. Silicone has been shown to increase collagenase activity and TGF β-2
levels and to regulate both cell proliferation and differentiation27.
This treatment should be performed after complete scar healing and for at least 12
hours a day for 2 to 3 months while maintaining a hygienic field6. A possible adverse reaction is folliculitis7. Silicone can be applied as sheets or gels, and both formulations are safe, effective,
and produce good aesthetic results28.
3.7 - Radiation therapy
Radiation therapy consists of applying ionizing radiation to keloids using different
therapeutic sessions and on different days. This therapy inhibits fibroblast proliferation
and stimulates fibroblast differentiation27. Several studies have demonstrated the high efficacy of this method in reducing keloid
recurrence when administered immediately after surgical excision29.
Radiotherapy as an adjuvant for operated keloids can be performed using different
techniques, including TGF-β therapy, conventional X-ray, single-dose radiotherapy,
and electron-beam radiotherapy. Electron irradiation is superior to conventional irradiation
because of its better dose distribution in the target tissue and less penetration
into deeper adjacent tissues30.
The dose used for treating keloids should be adapted according to the location of
the lesion, and the highest doses should be used at high-risk sites. The effectiveness
of this approach in keloid prevention depends on the biologically effective dose,
and no consensus has been reached on the best dosage. A minimum follow-up of 2.5 years
with postoperative electron irradiation is necessary to achieve satisfactory results
regarding scar quality and reduction of recurrence30.
A study analyzed the treatment of 174 ear lobe keloids using a total dose of 10 Gy
in 2 sessions or 15 Gy in 3 sessions. After 18 months of follow-up, the recurrence
rate was 4% with no significant difference between doses31. Another study found that the overall relapse rate was 5.6% after a mean follow-up
period of 40 months, and this rate was lower in patients who received doses higher
than or equal to 20 Gy32.
Electron irradiation is well-tolerated by patients. Nonetheless, potential adverse
effects include transient hyperpigmentation of the treated area and persistent peeling
for approximately 3 months. In addition, it should be used with caution in children
and young adults since a few body regions are sensitive to radiation in these populations.
A study reported the potential risk of malignancy of cells subjected to radiotherapy27.
3.8 - Pressure therapy
Pressure therapy is a prophylactic approach involving the application of continuous
pressure to keloids for several weeks to inhibit the development of new scars or to
reduce the size of pre-formed scars27,33. This therapy can be performed using compression bandages or elastic compression
stockings27. Pressure earrings, metal clips, and magnets, with or without silicone sheeting,
have been used for treating ear lobe keloids34.
The mechanism of action is not well established but seems to be related to the occlusion
of small blood vessels by pressure, leading to oxygen and nutrient deprivation and
consequently decreasing fibroblast proliferation and collagen synthesis. In addition,
decreased capillary blood flow reduces the level of alpha-2-macroglobulins resulting
in the inhibition of collagenases27,34.
The optimal applied pressure is difficult to determine. Notwithstanding, this value
should exceed capillary blood pressure, but should not decrease peripheral blood pressure
(20-30 mmHg). A prospective study evaluated 11 patients with keloids initially treated
with surgical resections, followed by pressure therapy after 15 to 21 days. Magnets
with pressures between 33 and 47 mmHg were used, and the recurrence-free rate was
90.91% at the end of a 4-6-month follow-up period34.
Nonetheless, evidence supporting the use of this technique is limited. A meta-analysis
that evaluated 6 high-quality studies showed no significant difference in the results
between burn patients treated with or without pressure therapy35. The therapy has no significant side effects, except for possible pressure discomfort27. Moreover, treatments are simple and affordable, and therefore, essential in regions
with limited technological and pharmacological resources27,34.
3.9 - Tissue adhesives (Prineo®)
Prineo® is a double polyester mesh system containing the glue octyl-2-cyanoacrylate
and belongs to the group of tissue adhesives. Molecular reactions begin when the adhesives
come into contact with the wound and the assembly adheres to keratinocytes36.
Prineo® should be applied to the margins of the incision as a thin layer in a single
movement. After application, the product dries in approximately 5 min and patients
may feel a warm sensation at the treatment site during the application37.
Tissue adhesives are applied quickly and easily, promote good tensile strength throughout
the wound, form an antimicrobial barrier, and cause less pain during removal. In addition,
scar enlargement using this method is smaller than that using suture closures. Prineo®
is recommended for extensive wounds with higher tensions36.
A study compared Prineo® and surgical sutures and found that there were no significant
differences (p > 0.05) in scar quality including aesthetics. In the study sample (n
= 101), there was only one reported case of a keloid associated with surgical sutures
and no cases associated with Prineo® (p = 0.042). The most relevant adverse effects
are contact dermatitis in response to the formaldehyde released during the polymerization
reaction between cyanoacrylate and the skin36.
A literature review has shown that this method is not widely used for treating keloids
but is an additional option. Therefore, further studies on the use of Prineo® for
keloid prevention and treatment are needed.
CONCLUSION
Several surgical and adjuvant therapies are available for treating keloids. However,
the number of high-quality studies on this topic is small and there is no consensus
on a universally accepted approach. Decisions regarding the best therapies are individualized
and vary according to the size of the lesion, lesion recurrence, and available medical
resources. Intralesional corticosteroid injections associated with other methods such
as silicone gel sheeting or pressure therapy produce good aesthetic results in smaller
lesions, whereas triamcinolone injections combined with cryotherapy yields good results
in larger lesions.
COLLABORATIONS
|
PMC
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Investigation, Methodology, Writing - Original Draft Preparation
|
|
CEFP
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Investigation, Methodology, Writing - Original Draft Preparation
|
|
EMS
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Investigation, Methodology, Writing - Original Draft Preparation
|
|
GASB
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Investigation, Methodology, Writing - Original Draft Preparation
|
|
JOJ
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Investigation, Methodology, Writing - Original Draft Preparation
|
|
LBO
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Investigation, Methodology, Writing - Original Draft Preparation
|
|
OCL
|
Analysis and/or data interpretation, Conception and design study, Conceptualization,
Investigation, Methodology, Writing - Original Draft Preparation
|
|
JSFL
|
Final manuscript approval, Supervision
|
REFERENCES
1. Pereira ALC, Leal F, Azulay DR, Azulay RD. Dermatologia. 6ª ed. Rio de Janeiro (RJ):
Guanabara Koogan; 2013.
2. Shin JY, Lee JW, Roh SG, Lee NH, Yang KM. A comparison of the effectiveness of triamcinolone
and radiation therapy for ear keloids after surgical excision: a systematic review
and meta-analysis. Plast Reconstr Surg. 2016 Jun;137(6):1718-25. PMID: 27219228 DOI:
https://doi.org/10.1097/PRS.0000000000002165
3. Goldstein BG, Goldstein AO. Keloids and hypertrophic scars. Dellavalle RP, Levy ML,
Corona R, editors. Post TW. Waltham, MA: UpToDate; 2019; [acesso em 2019 fev 16].
Disponível em: https://www.uptodate.com/contents/keloids-and-hypertrophic-scars
4. Khansa I, Harrison B, Janis JE. Evidence-based scar management. Plast Reconstr Surg.
2016 Sep;138(Suppl 3):165S-78S. DOI: https://doi.org/10.1097/PRS.0000000000002647
5. Gauglitz GG. Management of keloids and hypertrophic scars: current and emerging options.
Clin Cosmet Investig Dermatol. 2013;6:103-114. DOI: https://doi.org/10.2147/CCID.S35252
6. Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibe J, et al. Updated international
clinical recommendations on scar management: part 2 - algorithms for scar prevention
and treatment. Dermatol Surg. 2014 Aug;40(8):825-31.
7. Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids
and hypertrophic scars: a useful guide. Burns. 2014 Nov;40(7):1255-66. PMID: 24767715
DOI: https://doi.org/10.1016/j.burns.2014.02.011
8. Metsavaht LD. Abordagem cirúrgica de cicatrizes. Surg Cosmet Dermatol. 2016;8(1):11-20.
9. Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification,
and treatment. Dermatol Surg. 2017 Jun;43(Suppl 1):S3-S18. DOI: https://doi.org/10.1097/DSS.0000000000000819
10. Keeling BH, Whitsitt J, Liu A, Dunnick CA. Keloid removal by shave excision with adjuvant
external beam radiation therapy. Dermatol Surg. 2015 Aug;41(8):989-92. DOI: https://doi.org/10.1097/DSS.0000000000000417
11. Park TH, Park JH, Chang CH. Clinical features and outcomes of foot keloids treated
using complete surgical excision and full thickness skin grafting followed by corticosteroid
injections. J Foot Ankle Res. 2013;6:26. DOI: https://doi.org/10.1186/1757-1146-6-26
12. Rasheed I, Malachy A. The management of helical rim keloids with excision, split thickness
skin graft and intralesional triamcinolone acetonide. J Cutan Aesthet Surg. 2014 Jan/Mar;7(1):51-53.
DOI: https://doi.org/10.4103/0974-2077.129981
13. Verhiel S, Grzymala AP, van der Hulst R. Mechanism of action, efficacy, and adverse
events of calcium antagonists in hypertrophic scars and keloids: a systematic review.
Dermatol Surg. 2015 Dec;41(12):1343-50. DOI: https://doi.org/10.1097/DSS.0000000000000506
14. Danielsen PL, Rea SM, Wood FM, Fear MW, Viola HM, Hool LC, et al. Verapamil is less
effective than triamcinolone for prevention of keloid scar recurrence after excision
in a randomized controlled trial. Acta Derm Venereol. 2016 Aug;96(6):774-8.
15. Ahuja RB, Chatterjee P. Comparative efficacy of intralesional verapamil hydrochloride
and triamcinolone acetonide in hypertrophic scars and keloids. Burns. 2014 Jun;40(4):583-8.
PMID: 24182692 DOI: https://doi.org/10.1016/j.burns.2013.09.029
16. Ledon JA, Savas J, Franca K, Chacon A, Nouri K. Intralesional treatment for keloids
and hypertrophic scars: a review. Dermatol Surg. 2013 Dec;39(12):1745-57. PMID: 24299571
DOI: https://doi.org/10.1111/dsu.12346
17. Bijlard E, Steltenpool S, Niessen FB. Intralesional 5-fluorouracil in keloid treatment:
a systematic review. Acta Derm Venereol. 2015;95:778-782.
18. Wilson AM. Eradication of keloids: surgical excision followed by a single injection
of intralesional 5-fluorouracil and botulinum toxin. Can J Plast Surg. 2013;21(2):87-91.
DOI: https://doi.org/10.1177/229255031302100208
19. Pereima MJL, Goulart BC, Pereima RR, Feijó R, Freitas JL. Diminuição do tempo de maturação
de matrizes de regeneração dérmica quando associados a uso de curativos de pressão
negativa. Rev Bras Queimaduras. 2013;12(3):145-152.
20. Nguyen KT, Shikowitz L, Kasabian AK, Bastidas N. A novel approach to keloid reconstruction
with bilaminar dermal substitute and epidermal skin grafting. Plast Reconstr Surg.
2016 Jul;138(1):235-9. PMID: 26986991 DOI: https://doi.org/10.1097/PRS.0000000000002242
21. O’Boyle CP, Shayan-Arani H, Hamada MW. Intralesional cryotherapy for hypertrophic
scars and keloids: a review. Scars Burn Heal. 2017 Jan/Dec;3. DOI: https://doi.org/10.1177/2059513117702162
22. Bijlard E, Timman R, Verduijn GM, Niessen FB, Hovius SER, Mureau MAM. Intralesional
cryotherapy versus excision with corticosteroid injections or brachytherapy for keloid
treatment: Randomised controlled trials. J Plast Reconstr Aesthet Surg. 2018 Jun;71(6):847-856.
PMID: 29426811 DOI: https://doi.org/10.1016/j.bjps.2018.01.033
23. Brewin MP, Lister TS. Prevention or treatment of hypertrophic burn scarring: a review
of when and how to treat with the pulsed dye laser. Burns. 2014 Aug;40(5):797-804.
PMID: 24439925 DOI: https://doi.org/10.1016/j.burns.2013.12.017
24. Jin R, Huang X, Li H, Yuan Y, Li B, Cheng C, et al. Laser therapy for prevention and
treatment of pathologic excessive scars. Plast Reconstr Surg. 2013 Dec;132(6):1747-58.
PMID: 24281600 DOI: https://doi.org/10.1097/PRS.0b013e3182a97e43
25. Hultman CS, Friedstat JS, Edkins RE, Cairns BA, Meyer AA. Laser resurfacing and remodeling
of hypertrophic burn scars: the results of a large, prospective, before-after cohort
study, with long-term follow-up. Ann Surg. 2014 Sep; 260(3):519-29.
26. O’Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic
and keloid scars. Cochrane Database Syst Rev. 2013 Sep;1(9):CD003826. DOI: https://doi.org/10.1002/14651858.CD003826.pub3
27. Fernandes WS, Ferreira RCA. Queloide: uma revisão dos tratamentos atualmente disponíveis.
R Bras Ci Saúde. 2014;18(2):181-186. DOI: https://doi.org/10.4034/RBCS.2014.18.02.14
28. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic scars
and keloids. Journal of Cutaneous and Aesthetic Surgery. 2009 Jul/Dec;2(2):104-6.
DOI: https://doi.org/10.4103/0974-2077.58527
29. Lee SY, Park J. Postoperative electron beam radiotherapy for keloids: treatment outcome
and factors associated with occurrence and recurrence. Ann Dermatol. 2015 Feb;27(1):53-58.
DOI: https://doi.org/10.5021/ad.2015.27.1.53
30. Oliveira Júnior B, Schellini SA, Lastória JC, Carvalho LR, Stolf HO, Oliveira ALPD.
Tratamento de queloides usando radioterapia pósoperatória com elétrons: estudo comparativo
e randomizado com dois esquemas. Surg Cosmet Dermatol. 2013;5(1):16-26.
31. Ogawa R, Huang C, Akaishi S, Dohi T, Sugimoto A, Kuribayashi S, et al. Analysis of
surgical treatments for earlobe keloids: analysis of 174 lesions in 145 patients.
Plast Reconstr Surg. 2013 Nov;132(5):818e-825e. PMID: 24165633 DOI: https://doi.org/10.1097/PRS.0b013e3182a4c35e
32. Renz P, Hasan S, Gresswell S, Hajjar RT, Trombetta M, Fontanesi J. dose effect in
adjuvant radiotherapy for the treatment of resected keloids. Int J Radiat Oncol Biol
Phys. 2018 Sep;102(1):149-154. PMID: 29970316 DOI: https://doi.org/10.1016/j.ijrobp.2018.05.027
33. Monstrey S, Middelkoop E, Vranckx JJ, Bassetto F, Ziegler UE, Meaume S, et al. Updated
scar management practical guidelines: non-invasive and invasive measures. J Plast
Reconstr Aesthet Surg. 2014 Aug;67(8):1017-25. DOI: https://doi.org/10.1016/j.bjps.2014.04.011
34. Quintero-Larrovere M, Soto-Montenegro AE, Lozada-Urbani JA. Uso de imanes en el tratamiento
de queloides auriculares. Cir Plást Iberolatinoam. Madrid. 2017 Abr/Jun;43(2):163-174.
35. Anzarut A, Olson J, Singh P, Rowe BH, Tredget EE. The effectiveness of pressure garment
therapy for the prevention of abnormal scarring after burn injury: a meta-analysis.
J Plast Reconstr Aesthet Surg. 2009 Jan;62(1):77-84. PMID: 18249046 DOI: https://doi.org/10.1016/j.bjps.2007.10.052
36. Cammarota MC, Moura LG, Ribeiro Junior ISMAR, Lima RQD, Almeida CMD, Daher L, Daher
JC. Comparação de resultado estético em cicatrizes com uso de fios cirúrgicos e Prineo®.
Rev Bras Cir Plást. 2017;32(1):101-108.
37. Lipsett S. Minor wound repair with tissue adhesives (cyanoacrylates). Wolfson AB,
Wiley JF, editors. Post TW. Waltham, MA: UpToDate; 2019; [acesso em 2019 fev 16].
Disponível em: https://www.uptodate.com/contents/minor-wound-repair-with-tissue-adhesives-cyanoacrylates
1. Universidade Federal de Ouro Preto, Escola de Medicina, Ouro Preto, MG, Brazil.
Corresponding author: Pedro Martins Corrêa Rua Darcy Vargas, 40, Apto 102, Nova Suiça, Belo Horizonte, MG, Brazil. Zipe Code:
30421-093. E-mail: pedromc1987@gmail.com
Article received: December 19, 2018.
Article accepted: April 21, 2019.
Conflicts of interest: none.