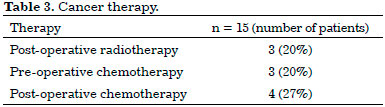ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Skin-reducing mastectomy using inferior pedicle dermal fat flaps in immediate reconstruction of medium and large hypertrophic breasts
Mastectomia redutora de pele com retalho dermogorduroso de pedículo inferior na reconstrução imediata de mamas com médias e grandes hipertrofias
ABSTRACT
INTRODUCTION: Breast cancer is the most common type of neoplasm among women (except for non-melanoma skin cancers), and in the past few years, its treatment greatly evolved with skin-sparing mastectomies. Breasts with grades II and III ptosis are difficult to approach and require an aesthetic reduction of the cutaneous envelope after adenomastectomy.
METHODS: A retrospective study was conducted from January 2013 to January 2016. This included all patients undergoing adenomastectomy and immediate reconstruction via reduction of the cutaneous envelope using Pitanguy's marking technique associated with the use of a prosthesis or expander above the submuscular plane and below the dermal fat flap.
RESULTS: A total of 15 patients were operated on based on the proposed technique, accounting for a total of 25 breasts (10 cases were bilateral). Twelve patients had cancer; two underwent prophylactic surgery; and one developed juvenile giant fibroadenoma. In two cases, an expander was used, and in 23 breasts, a definitive prosthesis was placed in a single surgical period. In three breasts, the nipple-areolar complex (NAC) was resected for oncological reasons; of the 22 preserved, 15 underwent grafting, and seven underwent elevation through the superior pedicle flap. There were five complications (20%): three seromas (12%), one necrosis at the "T" junction (4%), and one total NAC necrosis (4%).
CONCLUSION: The technique described is an effective and reproducible method of immediate breast reconstruction using implants in a single period in patients with large breasts. However, the right patients should be selected because the risks of complications cannot be neglected.
Keywords:
Breast neoplasms; Subcutaneous mastectomy; Mammoplasty; Surgical flaps; Breast Implants.
RESUMO
INTRODUÇÃO: O câncer de mama é o tipo mais comum entre as mulheres (excetuando-se os de pele não melanoma) e nos últimos anos seu tratamento evoluiu muito com as mastectomias preservadoras de pele. As mamas com ptose grau II e III são de difícil abordagem e necessitam da redução estética do envelope cutâneo após o procedimento de adenomastectomia.
MÉTODOS: Foi realizado um estudo retrospectivo de janeiro de 2013 a janeiro de 2016 em que foram incluídas todas as pacientes submetidas à adenomastectomia e reconstruídas imediatamente através de redução do envelope cutâneo com a técnica de marcação de Pitanguy associada ao emprego de prótese ou expansor no plano submuscular superiormente e abaixo do retalho dermicogorduroso inferiormente.
RESULTADOS: Um total de 15 pacientes foram operadas pela técnica proposta, contabilizando um total de 25 mamas (10 casos foram bilaterais). Doze pacientes apresentavam câncer, duas realizaram cirurgia profilática e uma apresentava fibroadenoma gigante juvenil. Em dois casos foi utilizado expansor e em 23 mamas foi colocada a prótese definitiva em um único tempo cirúrgico. Em três mamas o complexo aerolopapilar (CAP) foi ressecado por motivos oncológicos; dos 22 preservados, em 15 foi realizado enxerto e em 7 ascensão através de retalho de pedículo superior. Houve 5 casos de complicação (20%), sendo 3 seromas (12%), 1 necrose na junção do 'T' (4%), e 1 necrose total do CAP (4%).
CONCLUSÃO: A técnica descrita fornece um método eficaz e reproduzível de reconstrução mamária imediata com prótese em um tempo único em pacientes com mamas volumosas. Contudo, deve-se selecionar bem os pacientes, pois não se pode negligenciar os riscos de complicações.
Palavras-chave:
Neoplasias da mama; Mastectomia subcutânea; Mamoplastia; Retalhos cirúrgicos; Implantes de mama.
INTRODUCTION
Breast cancer is the most common type of neoplasm among women in Brazil and worldwide, after non-melanoma skin cancer, accounting for approximately 25% of new cases per year. According to INCA, the estimated number of new cases in 2016 in Brazil was 57,9601.
Breast cancer treatment evolved significantly over the last years. Early diagnosis and treatment are essential. For cases in which mastectomy became necessary, reconstruction options are already well established. Skin-sparing mastectomies greatly facilitated reconstruction methods to obtain aesthetic results in early-stage cases2,3. By sparing the cutaneous envelope and inframammary fold, a much more satisfactory result can be achieved during reconstruction4. The nipple-areolar complex (NAC) can also be preserved, when its maintenance does not compromise oncologic therapy protocols.
Reconstruction possibilities typically include the use of expanders or implants; pedicle flaps, usually the transverse rectus abdominis myocutaneous (TRAM) flap or the myocutaneous flap of the large dorsal muscle, or free flaps, such as the free TRAM flap and deep inferior epigastric artery perforator flap. Although reconstructions using autologous tissues yield a more satisfactory long-term outcome, reconstructions using implants or expanders are widely used, as these are simpler and faster procedures, yielding less surgical morbidity in patients4,5.
Large breasts are difficult to approach when proposing adenomastectomy, since the cutaneous envelope is large, and therefore, it is difficult to obtain a final aesthetic and harmonious result. Within this context, Bostwick6 first described a skin-reducing mastectomy procedure, which is performed via a classic reduction mammoplasty incision, "wise pattern," associated with the use of an implant or expander above the submuscular plane and below the dermal fat flap (DFF), resulting in an external inverted-T scar.
OBJECTIVE
The aim of this study is to report the applicability of skin-reducing mastectomy, which is performed via a classic Pitanguy's reduction mammoplasty incision, associated with the use of an implant or expander above the submuscular plane and below the DFF, resulting in an external inverted-T scar in the breasts with medium and large hypertrophies.
METHODS
A retrospective study was conducted. This included all patients who underwent adenomastectomy and immediate reconstruction via reduction of the cutaneous envelope using Pitanguy's marking technique and inferior pedicle DFF. The study was conducted from January 2013 to January 2016 on selected patients of the Breast Center of São Lucas Hospital of the Pontifical Catholic University of Rio Grande do Sul and in the private clinic of the author. The study followed the Declaration of Helsinki principles, and all patients provided their informed consent.
Patient data, such as age, smoking, and comorbidities, as well as the indication for surgery, including the type of tumor, and completion of chemotherapy and radiotherapy were reviewed.
Reconstruction characteristics, as well as complications, were also reviewed. Major complications were considered as those that evolved in a loss of reconstruction (implant removal); all other complications were considered minor.
To perform the technique described, the patients must have medium to large breasts with breast ptosis at least of grade II, but ideally with Regnault grade III. We used the criteria of Nava et al.4, which included pendulous breasts with a distance from the areola to the inframammary fold greater than 8 cm and a distance from the sternal furcula to the papilla greater than 25 cm.
The NAC was preserved only in cases where there was no macroscopic impairment or in which the retroareolar margins were negative in the intraoperative freezing.
Surgical technique
Preoperative marking was performed with the patient standing, via classic marking of Pitanguy's mammoplasty7 with some particularities. Initially, the mid-line mammary meridian, inframammary fold, and previous axillary line were marked. Point A was marked at the level of the inframammary fold, while points B and C were marked as close as possible to the nipple, keeping the distance AB and AC between 8 and 10 cm. A line joining point B to the inframammary fold medially and another line joining point C to the inframammary fold laterally were drawn (Figure 1).
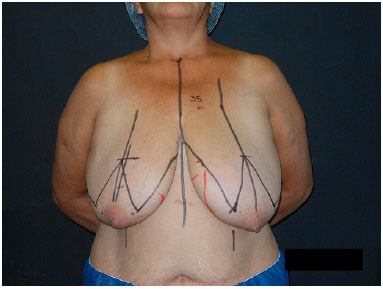 Figure 1.
Figure 1. Pre-operative markings based on Pitanguy's technique.
The surgery began with the de-epidermization of the entire lower pole of the breast (Figure 2), and when the NAC graft was planned, it was subsequently removed and placed in saline solution. Thereafter, the mastology team performed adenomastectomy, keeping the entire inferior pole flap as a DFF that will cover the entire inferior portion of the prosthesis (Figure 3).
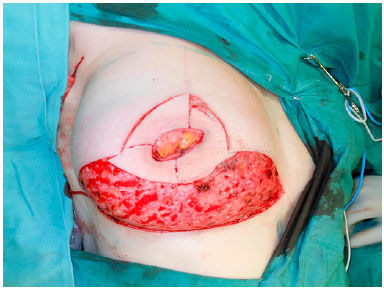 Figure 2.
Figure 2. Total breast pole de-epidermization.
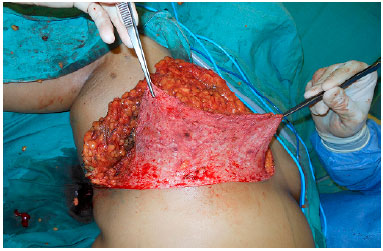 Figure 3.
Figure 3. Inferior dermal fat pedicle flap.
After completion of the oncological procedure, the lateral border of the pectoralis major muscle was identified, and the submuscular plane was created, taking out completely the muscle from its inferior costal insertion up to the fourth intercostal space at its sternal insertion. The DFF was then sutured to the pectoralis major muscle, while ensuring that the entire inferior portion of the implant was covered (Figure 4). The serratus muscle, mostly and when necessary, was dissected from the rib cage enough to cover the lateral portion of the implant and sutured to the lateral border of the pectoralis major pectoral muscle and DFF (Figure 5).
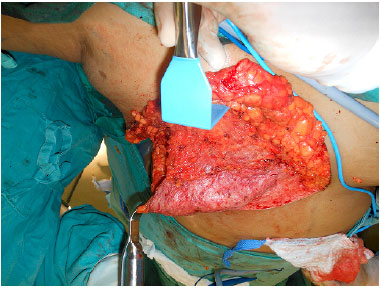 Figure 4.
Figure 4. Inferior dermal fat pedicle flap via suturing of the pectoralis major muscle.
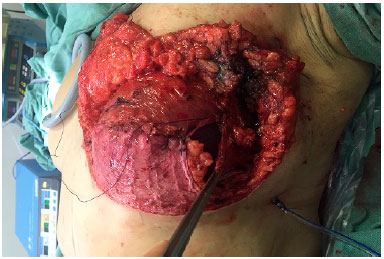 Figure 5.
Figure 5. Serratus anterior muscle assisting in covering the lateral portion of the implant.
The skin was closed, while suturing points B and C in the mammary meridian, thus repositioning the cutaneous envelope (Figure 6). When preserved, the NAC was supplied by a superior pedicle and maintained 4 cm from the inframammary fold in the midclavicular line. When NAC grafting was performed, the recipient area, also located at 4 cm from the inframammary fold, was de-epidermized, and the NAC was sutured to the site (Figure 7). Thereafter, Brown dressing was performed. The remaining incisions were sutured, and the resulting scar was shaped as an inverted "T." A suction drain (Porto-Vac) was routinely used inside the region with the implant.
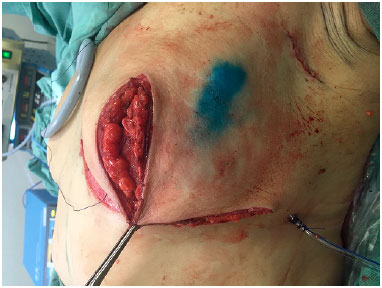 Figure 6.
Figure 6. Repositioning of the skin envelope by suturing points B and C in the mammary meridian.
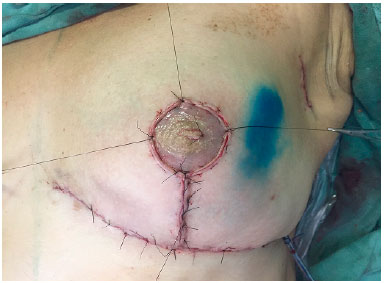 Figure 7.
Figure 7. Nipple-areolar complex graft: recipient area located 4 cm from the inframammary fold.
All patients used a surgical bra from the moment they exited the operating room until 1 month after surgery. Brown dressing was removed after 5 days, and the drain was maintained until the flow rate, with serous content, was lower than 30 mL in 24 hours.
RESULTS
A total of 15 patients were operated on based on the technique proposed, accounting for a total of 25 breasts (10 cases were bilateral). Twelve patients had cancer; two underwent prophylactic surgery; and one developed a juvenile giant fibroadenoma.
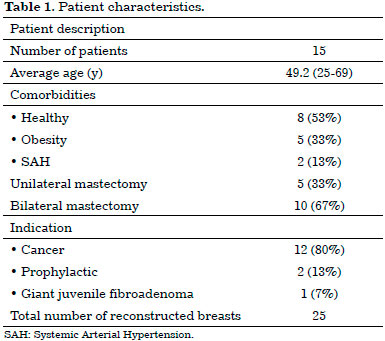
The mean age was 49.25 years (range, 25-69 years). Eight patients were healthy, and seven presented comorbidities. Two patients were controlled hypertensive, while five were obese (Table 1).
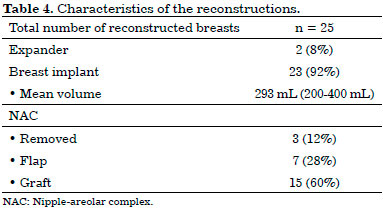
In the first two cases, which were unilateral, the expanders were placed and then replaced in a second surgical period with breast implants. In all other breasts, a definitive implant was placed in a single surgical period, accounting for a total of 23 breast implants. All implants were round, textured, and of a high profile, with a mean volume of 293 mL (range, 200-400 mL) (Table 2).
In three breasts, the NAC was resected for oncological reasons. Of the 22 preserved, 15 underwent grafting, and seven underwent elevation through a superior pedicle flap (Table 2).
Regarding the complementary treatment, three patients underwent neoadjuvant chemotherapy; four, adjuvant chemotherapy; and three, adjuvant radiotherapy (Table 3).
As for the pathological diagnosis, there were 10 invasive ductal carcinomas, three invasive lobular carcinomas, and two giant juvenile fibroadenomas; further, 10 cases underwent prophylactic mastectomy (Table 4).
The five patients who underwent unilateral procedure developed an invasive ductal carcinoma. Of the 10 patients who underwent a bilateral procedure, one presented giant juvenile fibroadenoma; two underwent bilateral prophylactic surgery; four had invasive ductal carcinoma in one breast and underwent prophylactic contralateral surgery; two presented invasive lobular carcinoma in one breast and underwent prophylactic contralateral surgery; and one presented invasive lobular carcinoma in one breast and contralateral invasive ductal carcinoma.
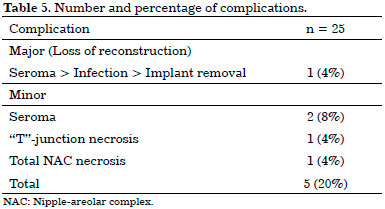
There were five complications (20%): three seromas (12%), one necrosis at the "T"-junction (4%), and one total NAC necrosis (4%). Of these cases, one has been considered a major complication, since it evolved into loss of reconstruction (case cited below), and four were considered minor complications (Table 5).
Regarding seroma cases, the case of the first patient evolved to infection, requiring the removal of the implant for a period of 6 months and subsequent reconstruction using the latissimus dorsi flap associated with the implant (major complication). The case of the second patient evolved to dehiscence of the surgical wound with prosthesis extrusion; thus, the patient was brought to the operating room to wash the space, remove the extruded implant, and replace it with a new one. The third patient demanded an intervention owing to the chronicity of the seroma, which persisted after multiple ultrasound-guided punctures. She was then brought to the operating room to wash the cavity and replace the implant with a Porto-Vac suction drain insertion. All these cases evolved well after the interventions mentioned above.
The patient who presented necrosis at the "T"-junction was treated with debridement and suturing in the ambulatory setting. The patient who had total NAC necrosis underwent debridement and total skin grafting (Figure 8). All NAC grafts integrated 100% at the expense of some flattening of the nipple and some degree of depigmentation (Figure 9).
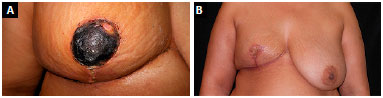 Figure 8. A:
Figure 8. A: Total necrosis of the nipple-areolar complex;
B: Final aspect after debridement and total skin grafting.
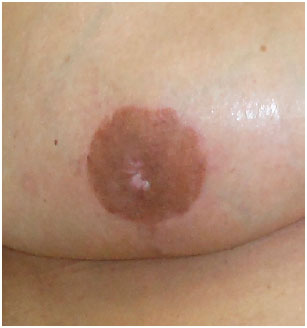 Figure 9.
Figure 9. Final appearance of the nipple-areolar complex graft (to note the flattening of the nipple and some degree of depigmentation).
DISCUSSION
The term "skin-sparing mastectomy" was introduced by Toth and Lappert, apud Hammond et al.8, in 1991, who suggested the realization of a mastectomy with the preservation of the cutaneous envelope for better aesthetic results of the operated breast. Oncological safety in terms of local recurrence has already been documented by several authors3,9-15.
Carlson et al.3 described four types of skin-sparing mastectomy, which are based on the type of incision and amount of excised skin. Types I and III are used in small breasts with mild ptosis through periareolar incisions. Type IV is used in large breasts presenting significant ptosis, which require aesthetic resection of the cutaneous envelope.
Regnault16 classified the degree of breast ptosis as grade II breasts in which the nipple is slightly below the inframammary fold, although the lower pole is still visible and as degree III breasts in which the nipple is well below the inframammary fold, and the lower visible pole is not visible anymore. These patients with large breasts, recommended for skin-sparing mastectomy, benefit greatly from a reconstruction with aesthetic skin resection and implant coverage with a DFF of the lower pedicle and large pectoralis muscle.
The technique previously described by Nava et al.13 in 2006 and named "skin-reducing mastectomy" offers a suitable space to place the implant, without excess tension and with a decreased risk of breast prosthesis extrusion in case of skin flap necrosis, as covered with autologous tissues. Placing the implant in a region covered with well-vascularized tissues is safer and subsequently provides comfort to the plastic surgeon5. In case of skin necrosis, which occurs as frequent as 27% in the "T"-junction, the treatment consists only of dressings15. In our series, we had one case of necrosis at the "T"-junction, which was re-sutured with local anesthesia in the outpatient setting.
Hammond et al.8 reported the realization of this surgery through a two-stage reconstruction using an expander. In our series, we used an expander in the first two cases, owing to the lack of experience and credibility of flap perfusion. However, it was noted that the space was extremely suitable and safe for the reconstruction in a single surgical period using a definite breast implant.
Placing a breast implant in a space only with the pectoralis major muscle results in an inadequate coverage of its lower portion, as we often cover only with subcutaneous tissues in this area. With the technique used, we achieved a full coverage of the implant using autogenous tissues, without the need for materials, such as tissues or acellular dermal matrix (ADM), to cover the inferolateral portion of the implant. The use of these allogeneic substances increases the costs of the surgery, besides having their own complications. In addition, using ADMs is not yet allowed in Brazil.
With the described technique, the implant does not have the tendency to migrate to the upper pole, since we have enough space in the lower pole for its accommodation. Thus, the implant undergoes a natural and desirable ptosis over time8. In addition, the larger the breast of the patient in the preoperative period, the greater the inferior flap obtained, thus allowing creation of a space for the direct insertion on the right side of a large implant without restricting its projection. This eliminates the need for a tissue expander. In our experience, we were able to use implants up to 400 mL in a space with an adequate volume, without causing tension in the adjacent tissues.
The number of complications related to this surgery is acceptable in view of the relative complexity of the operative procedure. Hudson and Skoll5 reported a complication rate of 15.7% in 19 operated breasts. Hammond et al.8 reported a complication rate of 16.6% in 12 operated breasts while using an expander. Nava et al.13 reported a complication rate of 33.3% in 30 operated breasts. Ladizinsky et al.17 reported a complication rate of 24% in 170 operated breasts. De Vita et al.18 reported a complication rate of 25% in 88 operated breasts.
In our series, we had a complications rate of 20% in 25 operated breasts, and seroma was the most common complication. This occurred despite the use of routine vacuum suction drains, which were kept in a conservative manner until the flow rate with serous content was lower than 30 mL in 24 hours. All these seroma cases evolved into surgical treatment; however, only one evolved with loss of reconstruction, requiring a reconstructive surgery with the latissimus dorsi muscle flap associated with the implant 11 months after the removal of the initial implant. In the other seroma cases, surgery was performed to wash the cavity, replace the implant, and place the suction drain. Both cases evolved satisfactorily.
In the first cases, we elevated the NAC using superior pedicle flaps. However, owing to a doubt concerning its adequate vascularization in the trans-operative period and after the first case of total NAC necrosis, we began to graft all NACs when they can be preserved from a cancer point of view. As in the study by King et al.19, we removed all the retroareolar tissues until the dermis and sent the material for freezing and definitive anatomopathological analysis to obtain a greater oncological safety. With this technique, we obtained satisfactory results without any NAC loss, although at the expense of flattening the nipple and possibility of NAC discoloration.
The classic Pitanguy's marking for reduction mammoplasty yields safety and reliability of the procedure in cases requiring symmetrization of the opposite breast, since the cutaneous marking of one breast can be easily transposed to the other, resulting in an adequate symmetry during reconstruction. In all unilateral cases, the symmetrization was performed in the other breast using the classic Pitanguy's method7, resulting in relatively symmetric breasts (Figures 10 and 11).
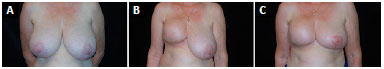 Figure 10. A:
Figure 10. A: Pre-operative image of a patient with large breasts, presenting grade III ptosis;
B: One-year and 9-month post-operative image of the right skin-reducing mastectomy with the insertion of a 325-mL round and highprofile implant;
C: Image 2 years after skin-reducing mastectomy and opposite breast symmetrization using Pitanguy's technique.
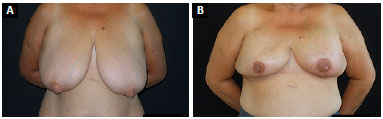 Figure 11. A:
Figure 11. A: Pre-operative image of a patient with very pendulous breasts, presenting grade III ptosis;
B: Six-month post-operative image of bilateral skin-reducing mastectomy with nipple-areolar complex grafting and insertion of a 400-mL round and high-profile implant.
CONCLUSION
Currently, the surgical treatment of breast cancer must be well planned to achieve an aesthetically satisfactory result. The described skin-reducing mastectomy technique, which was performed via a Pitanguy's classic reduction mammoplasty incision associated with the use of an implant or expander above the submuscular plane and below the DFF, resulting in an external inverted-T scar, is an effective and reproducible method of immediate breast reconstruction in patients with medium and large hypertrophic breasts. A full coverage of the implant using autogenous tissues can be achieved. This confers protection to the surgical wound in its lower pole through the use of a DFF, besides allowing the use of large implants without restricting its projection.
COLLABORATIONS
FFL Analysis and/or interpretation of data; statistical analyses; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
REFERENCES
1. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva. Rio de Janeiro: Inca; 2016. [acesso 2016 Abr 16]. Disponível em: www2.inca.gov.br
2. Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87(6):1048-53. PMID: 1852020 DOI: http://dx.doi.org/10.1097/00006534-199106000-00006
3. Carlson GW, Bostwick J 3rd, Styblo TM, Moore B, Bried JT, Murray DR, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225(5):570-5. DOI: http://dx.doi.org/10.1097/00000658-199705000-00013
4. Nava MB, Ottolenghi J, Pennati A, Spano A, Bruno N, Catanuto G, et al. Skin/nipple sparing mastectomies and implant-based breast reconstruction in patients with large and ptotic breast: oncological and reconstructive results. Breast. 2012;21(3):267-71. DOI: http://dx.doi.org/10.1016/j.breast.2011.01.004
5. Hudson DA, Skoll PJ. Complete one-stage, immediate breast reconstruction with prosthetic material in patients with large or ptotic breasts. Plast Reconstr Surg. 2002;110(2):487-93. DOI: http://dx.doi.org/10.1097/00006534-200208000-00018
6. Bostwick J. Prophylatic (risk reducing) mastectomy and reconstruction. In: Bostwick J, ed. Plastic and Reconstructive Breast Surgery. St. Louis: Quality Medical Publishing; 1990. p. 1369-73.
7. Pitanguy I. Surgical treatment of breast hypertrophy. Br J Plast Surg. 1967;20(1):78-85. PMID: 5339723 DOI: http://dx.doi.org/10.1016/S0007-1226(67)80009-2
8. Hammond DC, Capraro PA, Ozolins EB, Arnold JF. Use of a skin-sparing reduction pattern to create a combination skin-muscle flap pocket in immediate breast reconstruction. Plast Reconstr Surg. 2002;110(1):206-11. PMID: 12087255 DOI: http://dx.doi.org/10.1097/00006534-200207000-00035
9. Kroll SS, Ames F, Singletary SE, Schusterman MA. The oncologic risks of skin preservation at mastectomy when combined with immediate reconstruction of the breast. Surg Gynecol Obstet. 1991;172(1):17-20. PMID: 1985335
10. Carlson GW, Styblo TM, Lyles RH, Jones G, Murray DR, Staley CA, et al. The use of skin sparing mastectomy in the treatment of breast cancer: The Emory experience. Surg Oncol. 2003;12(4):265-9. DOI: http://dx.doi.org/10.1016/j.suronc.2003.09.002
11. Carlson GW, Styblo TM, Lyles RH, Bostwick J, Murray DR, Staley CA, et al. Local recurrence after skin-sparing mastectomy: tumor biology or surgical conservatism? Ann Surg Oncol. 2003;10(2):108-12. PMID: 12620903
12. Gerber B, Krause A, Reimer T, Müller H, Küchenmeister I, Makovitzky J, et al. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg. 2003;238(1):120-7. PMID: 12832974 DOI: http://dx.doi.org/10.1097/01.SLA.0000077922.38307.cd
13. Nava MB, Cortinovis U, Ottolenghi J, Riggio E, Pennati A, Catanuto G, et al. Skin-reducing mastectomy. Plast Reconstr Surg. 2006;118(3):603-10. DOI: http://dx.doi.org/10.1097/01.prs.0000233024.08392.14
14. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227-32. PMID: 12393819 DOI: http://dx.doi.org/10.1056/NEJMoa020989
15. Derderian CA, Karp NS, Choi M. Wise-pattern breast reconstruction: modification using AlloDerm and a vascularized dermal-subcutaneous pedicle. Ann Plast Surg. 2009;62(5):528-32. DOI: http://dx.doi.org/10.1097/SAP.0b013e3181a0cfee
16. Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg. 1976;3(2):193-203.
17. Ladizinsky DA, Sandholm PH, Jewett ST, Shahzad F, Andrews K. Breast reconstruction with the Bostwick autoderm technique. Plast Reconstr Surg. 2013;132(2):261-70. PMID: 23897325 DOI: http://dx.doi.org/10.1097/pRS.0b013e3182958774
18. De Vita R, Pozzi M, Zoccali G, Costantini M, Gullo P, Buccheri EM, et al. Skin-reducing mastectomy and immediate breast reconstruction in patients with macromastia. J Exp Clin Cancer Res 2015;34:120. DOI: http://dx.doi.org/10.1186/s13046-015-0227-5
19. King IC, Harvey JR, Bhaskar P. One-stage breast reconstruction using the inferior dermal flap, implant, and free nipple graft. Aesthetic Plast Surg. 2014;38(2):358-64. PMID: 24477522 DOI: http://dx.doi.org/10.1007/s00266-014-0276-8
1. Sociedade Brasileira de Cirurgia Plástica, Porto Alegre, RS, Brazil
2. Centro de Mama, Hospital São Lucas, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil
Institution: Hospital São Lucas, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Corresponding author:
Francisco Felipe Laitano
Avenida Ipiranga, Conjunto 520 - Jardim Botânico
Porto Alegre, RS, Brazil - Zip Code 26599-000
E-mail: fflpoa@hotmail.com
Article received: July 31, 2016.
Article accepted: July 10, 2017.
Conflicts of interest: none.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license