ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Preoperative and postoperative assessment of external nasal valve in rhinoplasty
Avaliação pré e pós-operatória da válvula nasal externa em rinoplastia
ABSTRACT
INTRODUCTION: The external nasal valve is located on the rim of the nostrils and is composed of soft tissues and cartilage. Any imbalance between these structures always leads to external nasal valve insufficiency, which may be partial or total, depending on the degree of alteration. The external nasal valve was evaluated before and after rhinoplasty to assess the efficiency of the technique used.
METHODS: This is a retrospective study that included 34 patients operated at private hospitals. The collapse of the nasal alae during deep inspiration and the vector of the lower lateral cartilage were evaluated. To provide more structure to the nasal alae, the lateral crural strut graft and/or alar contour graft were used.
RESULTS: The inappropriate vector of the lower lateral cartilage is related to the external nasal valve insufficiency (p = 0.006), which was corrected with grafts providing nasal alae remodeling in most of the cases (p = 0.006). A significant difference was observed in the frequency of using grafts in primary (66%) and secondary rhinoplasty (80%).
CONCLUSION: The inappropriate vector of the lower lateral cartilages usually resulted in an unstructured nasal alae, presenting external nasal valve insufficiency. Structuring the nasal alae with a lateral crural strut graft and/or alar contour graft was proven effective to correct external nasal valve insufficiency in 90% of the cases and to provide better aesthetic proportions and nasal contour. The frequency of grafts used in secondary rhinoplasty (80%) was higher than that in primary (66%), which indicates the need for grafts in a more complex surgery.
Keywords:
Nose; Nasal cartilage; Nasal obstruction; Rhinoplasty.
RESUMO
INTRODUÇÃO: A válvula nasal externa está localizada no rebordo das narinas e é composta por tecidos moles e cartilagem. Caso haja algum desequilíbrio entre estas estruturas, invariavelmente ocorrerá insuficiência desta válvula nasal externa, podendo ser parcial ou total, dependendo do grau das alterações. Avaliou-se a válvula nasal externa no pré e pós-operatório em rinoplastia verificando a eficiência da técnica utilizada.
MÉTODOS: Estudo retrospectivo de 34 pacientes operados em hospitais particulares. Foram avaliados o colabamento da asa nasal durante a inspiração profunda e o vetor das cartilagens laterais inferiores. Para estruturação da asa nasal, utilizou-se o enxerto de suporte da cruz lateral e/ou enxerto de contorno alar.
RESULTADOS: O vetor não adequado da cartilagem lateral inferior está relacionado com a insuficiência da válvula nasal externa (p = 0,006) e a estruturação da asa nasal, mediante o uso de enxertos, corrigiu esta afecção na maioria dos casos (p = 0,006). Houve diferença na frequência de utilização de enxertos na rinoplastia primária (66%) e secundária (80%).
CONCLUSÃO: O vetor inadequado das cartilagens laterais inferiores geralmente resultou em uma asa nasal desestruturada, com insuficiência da válvula nasal externa. A estruturação da asa nasal com enxerto de suporte da cruz lateral e/ou enxerto de contorno alar se mostrou eficaz na correção da insuficiência da válvula nasal externa em 90% dos casos, além de conferir proporções e contornos mais belos ao nariz. A frequência de utilização dos enxertos na rinoplastia secundária (80%) foi maior do que na primária (66%) e nos mostrou a necessidade dos enxertos numa cirurgia mais complexa.
Palavras-chave:
Nariz; Cartilagens nasais; Obstrução nasal; Rinoplastia.
INTRODUCTION
When humans evolved, nasal airflow was redirected from the olfactory mucosa to the nasal valves. Thus, the mucosa underwent a gradual atrophy, as it is located only in the cribiform plate, and nasal valves acquired a greater functional importance for breathing1.
The nasal valves regulates the entry of air through the nose and are of two types, one internal and the other external, one on each side. The internal nasal valve is located in the middle third of the nose and is the point of greatest narrowing for airflow, from the nostril to the lung. Its borders are as follows:
• medial: nasal septum;
• lower: nasal floor;
• lateral: lower turbinate;
• upper: upper lateral cartilage1-5.
The external nasal valve (ENV) is located on the rim of the nostrils and is similar to a rectangular triangle with a lower base. It has three walls as follows (Figure 1):
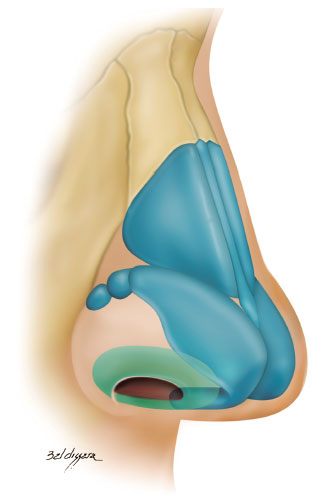 Figure 1.
Figure 1. External nasal valve (green).
• lateral: lateral crus of the lower lateral cartilage and fibroadiposal tissue of the nasal alae;
• medial: membranous septum and medial crus of the lower lateral cartilage;
• lower: nostril floor1-10.
The proper functioning of this valve relies on a correct anatomical and functional relationship of the structures previously mentioned. If these relationships are imbalanced, external nasal valve insufficiency always occurs. This may be partial or total in accordance with the degree of alterations7-12.
The ideal anatomical configuration of these structures will relate to ideal aesthetic features13. An important concept in modern rhinoplasty was introduced by Sheen5,6,14-16 and reviewed in detail by Gunter and Friedman6. These authors classified the vector of the lower lateral cartilage (a straight line drawn between the domus and the insertion site of this cartilage in the pyriform aperture) and enabled its repositioning and/or reinforcement, as well as its relationship with external nasal valve insufficiency.
To assess the functioning of external nasal valves, it is necessary to perform a dynamic physical examination, asking the patient to take a deep inspiration through the nose and then an expiration through the mouth. This will allow observing nasal alae movement and, subsequently, their function3,4.
The functional importance of nasal valves1-12,14-16, particularly the external nasal valve, was revealed by Constantian in 1994, when he evaluated with preoperative and postoperative rhinomanometry the separate reconstruction of external and internal nasal valves, and noticed a 2.6 and 2.0 times increase in nasal airflow, respectively. When he associated the treatment of septal deviation with the reconstruction of external and internal nasal valves, nasal airflow increased 4.9 times. All these results were statistically significant4.
The importance of nasal functioning has been the object of greater studies only in the last 20 years. However, few literatures are available, mainly in our environment.
OBJECTIVE
The aim of this study was to evaluate the external nasal valve before and after rhinoplasty as follows: analyze the preoperative relationship between the lower lateral cartilage vector and the external nasal valve insufficiency; examine, in the postoperative period, the relationship between a structured nasal alae (with grafts) and external nasal valve insufficiency; and to verify the frequency of grafts used in primary or secondary rhinoplasty.
METHODS
A retrospective study, which included 34 patients who underwent surgery at private hospitals, in the city of Ponta Grossa, PR, between March 2013 and January 2016. Of these, 25 were women (74%) and 9 were men (26%). The age varied between 18 and 73 years.
The present study was approved by the ethics committee of Ponta Grossa Faculties with the opinion number 1.969.050.
Routine preoperative and postoperative rhinoplasty photographic documentation was performed as follows: frontal, oblique, profile, mentonasal statics and dynamics (deep inspiration), and smiling (frontal and profile), and a movie was recorded to evaluate the function of external valves.
All preoperative patients who underwent a thorough examination of the external nasal valve were evaluated as follows: resistance to the collapse of the nasal alae during a deep inspiration3 and lower lateral cartilage vector9,10. The collapse of the nasal alae (Figure 2) was classified into the following based on the degree of external nasal valve insufficiency:
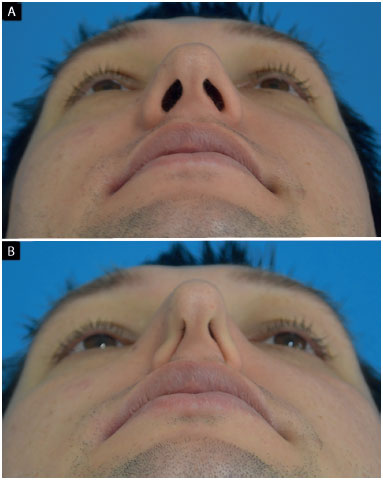 Figure 2.
Figure 2. Evaluation of nasal alae collapse during a deep inspiration.
A: Mentonasal statics;
B: Mentonasal dynamics (deep inspiration). Detail: bilateral total external nasal valve insufficiency.
• total: nasal alae touches the columella;
• partial: reduction of the horizontal axis of the nostril, without the nasal alae touching the columella;
• absent: absence of change in the horizontal axis of the nostril.
Regarding the vector of the lower lateral cartilages, in a frontal picture, a line was drawn between the domus and the site of insertion of this cartilage into the pyriform aperture. Its projection was then evaluated in relation to the pupil9,10.
When this line was medially directed toward the pupil, the vector was considered inappropriate and, in cases where this line was laterally directed to or matched the pupil, the vector was considered appropriate (Figure 3)9,10.
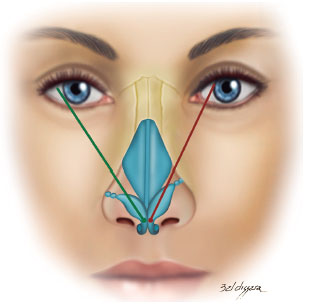 Figure 3.
Figure 3. Vector of lower lateral cartilages.
Note: Green: appropriate and red: not appropriate.
All the patients were operated under general anesthesia and local infiltration with vasoconstrictor. Anesthetic infiltration consisted of 2% lidocaine (Cristália®), 1% ropivacaine (Cristália®), 0.9% saline (Baxter®), and epinephrine 1:1.000 (Hipolabor®) at a concentration of 1:200.000.
The surgical technique of choice was open structure rhinoplasty with a stair-step columellar incision8 and two marginal incisions, with its distance of 5 mm from the nasal alae rim. The undermining was performed as follows: lower lateral cartilages in the sub-SMAS plane to keep intact the pyriform ligament and to not damage this structure, which is important for structuring the nasal alae17; upper lateral cartilages in the subperichondrial plane; and nasal bones in the subperiostal plane to avoid dorsum irregularities13.
Surgical sutures were routinely used as follows: cartilage grafts were fixed with PDS 5.0 or mononylon 5.0 (Ethicon®), marginal incisions were sutured with catgut 5.0 (Ethicon®) and skin columella with mononylon 6.0 (Ethicon®).
After surgery, a micropore tape was used on the dorsum and lateral wall of the nose and a thermo-moldable dressing was placed for 15 days. A gauze moistened with Nebacetin® was used for anterior nasal tamponade until the patient was discharged the following day.
The preferred donor area for cartilage grafts was the nasal septum, and in cases requiring more cartilage, conchal and costal cartilages were chosen6,8.
The surgical procedure was selected for each case and depended on the preoperative examination and the availability of the cartilage to perform the grafts. A floating columellar strut graft18,19 was routinely used to strengthen the medial wall of the external nasal valve. When necessary, the internal nasal valve, deviation of the cartilaginous septum, ethmoid spurs, maxillary crest, inferior turbinate hypertrophy, and middle bullous concha were carried out2-4.
The choice of the grafts for the treatment or prevention of nasal external valve insufficiency varied according to the availability of autologous material (mainly in secondary rhinoplasty), anatomical characteristics of the patient, vector of lower lateral cartilages, and severity of external nasal valve insufficiency.
We used the following grafts:
• lateral crural strut graft: the entire length of the lateral crus of the lower lateral cartilage was strengthened with a graft measuring 4-6 x 15-25 mm (Figure 4)6,18.
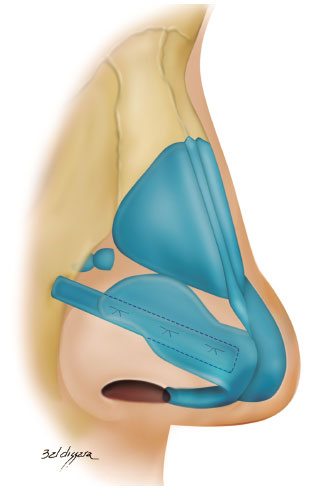 Figure 4.
Figure 4. Lateral crural strut graft.
In cases where the lateral crus was disinserted, its lateral reinsertion was fixed to the fibroadiposal tissue of the nasal alae, below the pyriform aperture (Figure 5), so it cannot be palpable, thus preventing the risk of fracture over the years6,18;
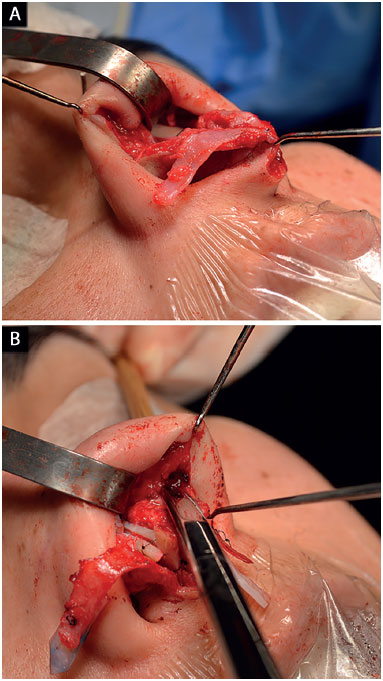 Figure 5.
Figure 5. Repositioning of the lower lateral cartilages.
A: Cartilage disinsertion;
B: Creation of the pocket to reinsert the cartilages with the appropriate vector.
• alar contour graft: caudally, we strengthened the nasal alae around the sideline with a graft measuring 3-4 x 15-20 mm and positioned it caudally to the marginal incision (Figure 6)8,18,20,21.
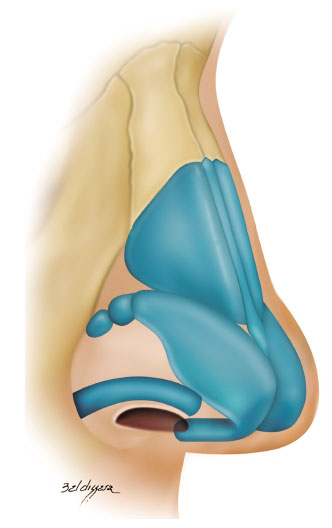 Figure 6.
Figure 6. Alar contour graft.
The pocket for the insertion of this graft was created with a 2 mm osteotome in the nasal alae contour, which extended from the most lateral portion of the nasal alae until the soft triangle (Figure 7)8,18,20,21.
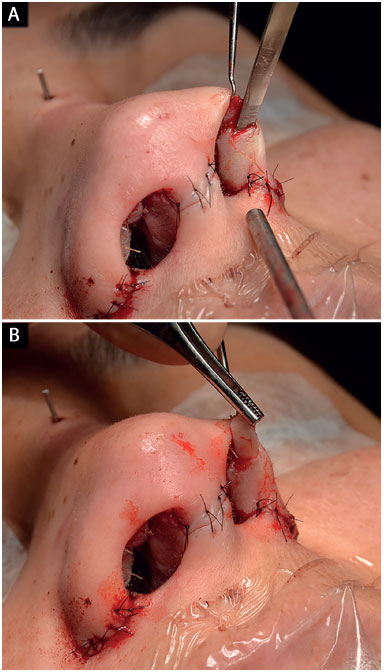 Figure 7.
Figure 7. Surgical technique for the alar contour graft.
A: Creation of the pocket with a 2 mm osteotome;
B: Graft insertion.
RESULTS
Thirty-four cases of rhinoplasty were performed between March 2013 and January 2016. The postoperative follow-up was between 6 and 34 months. Surgical revision was required only in two cases (6%), as an intense scar retraction caused the lack of nasal tip definition. Complications related to the use of the grafts were not observed.
The results were evaluated by using the chi-square and Fisher exact tests. P values of < 0.05 indicated statistical significance. The data were analyzed with the computer software IBM SPSS Statistics v.20.
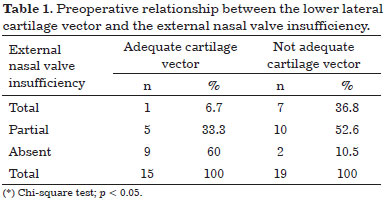
Preoperative relationship between the vector of the lower lateral cartilage and the external nasal valve insufficiency
The inappropriate vector of lower lateral cartilages in the preoperative period was statistically significant owing to the development of external nasal valve insufficiency (p = 0.006). The results obtained are shown in Table 1.
In Table 1, 6.7% of the cases that presented an appropriate vector were shown to develop total insufficiency of the external nasal valve. This percentage was 36.8% for cases with inappropriate vector. We also observed that 60% of the cases with an appropriate vector did not present external nasal valve insufficiency, while this percentage was 10.5% for cases with inappropriate vector.
The results obtained are shown in Figure 8.
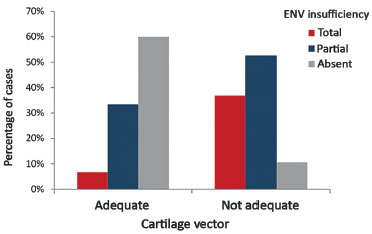 Figure 8.
Figure 8. Preoperative relationship between the lower lateral cartilage vector and the external nasal valve insufficiency.
ENV: External Nasal Valve.
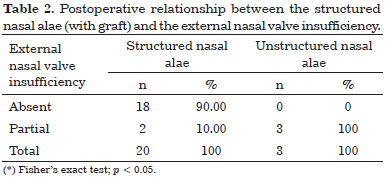
Postoperative relationship between a structured nasal alae (with grafts) and external nasal valve insufficiency
* Only 23 cases (68%) with external nasal valve insufficiency were considered. The remaining 11 cases (32%) that did not present external nasal valve insufficiency did not develop this affection in the postoperative period.
A structured nasal alae (with grafts) was statistically significant for the correction of external nasal valve insufficiency (p = 0.006). The results obtained are shown in Table 2.
In Table 2, all the cases with unstructured nasal alae are shown to present partial nasal external valve insufficiency after surgery. Among 20 cases with a structured nasal alae, only two (10%) presented partial nasal external valve insufficiency. The others did not present external nasal valve insufficiency.
The results obtained are shown in Figure 9.
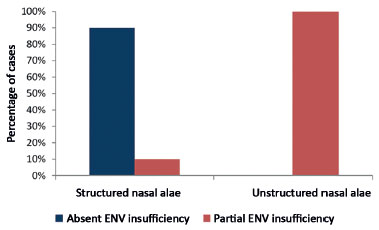 Figure 9.
Figure 9. Postoperative relationship between structured nasal alae (with grafts) and external nasal valve insufficiency.
ENV: External Nasal Valve.
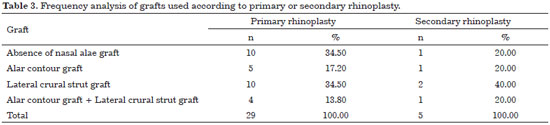
Frequency analysis in using grafts according to primary or secondary rhinoplasty
* All 34 cases were considered.
The lateral crural strut graft was used in 50% of the cases, followed by the alar contour graft, used in 29% of the cases. The use of both grafts in the same patient only occurred in 15% of the cases. Table 3 describes the frequencies and percentages of each graft according to primary or secondary rhinoplasty.
The following are cases with external nasal valve insufficiency and the treatment that was performed.
Case 1: Inappropriate vector of the lower lateral cartilages and partial left external nasal valve insufficiency. Surgery: lowering of the dorsum, discreet nasal shortening and bilateral lateral crural strut graft (without changing the vector of lower lateral cartilages; Figure 10).
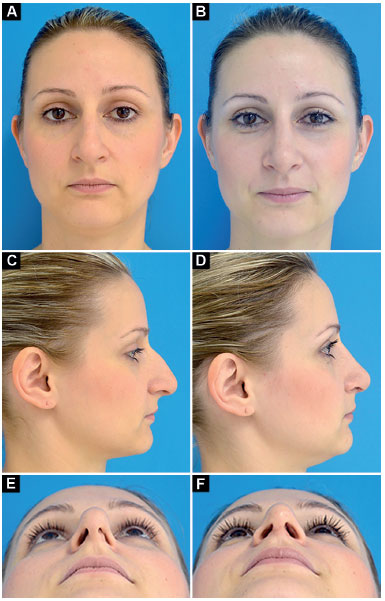 Figure 10.
Figure 10. Preoperative and postoperative period of 9 months.
A-B: Frontal;
C-D: Profile;
E-F: Deep inspiration.
Case 2: Inappropriate vector of the lower lateral cartilages and total bilateral external nasal valve insufficiency. Surgery: discreet nasal shortening and bilateral lateral crural strut graft (without changing the vector of lower lateral cartilages; Figure 11).
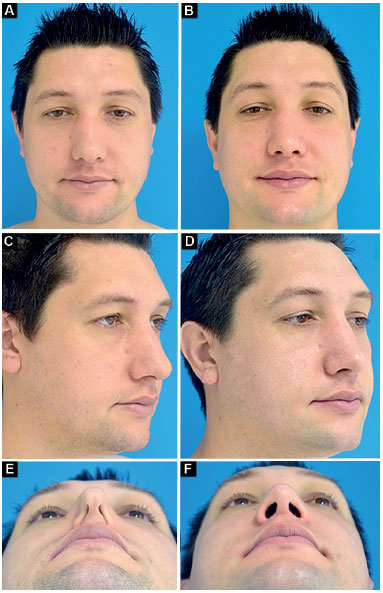 Figure 11.
Figure 11. Preoperative and postoperative period of 1 year.
A-B: Frontal;
C-D: Oblique;
E-F: Deep inspiration.
Case 3: Inappropriate vector of the lower lateral cartilages and total right and partial left external nasal valve insufficiency. Surgery: lowering of the dorsum, nasal shortening, bilateral lateral crural strut graft (changing the vector of lower lateral cartilages) and bilateral alar contour graft (Figure 12).
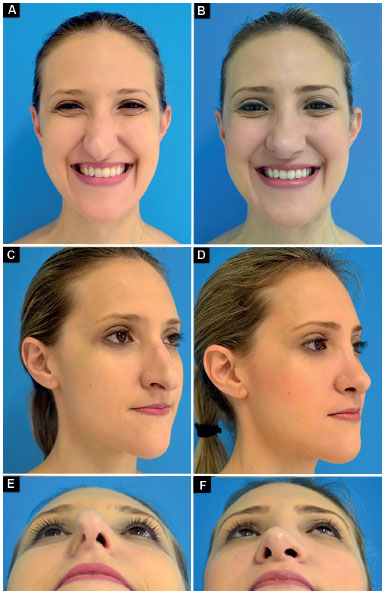 Figure 12.
Figure 12. Preoperative and postoperative period of 6 months.
A-B: Frontal smiling;
C-D: Oblique;
E-F: Deep inspiration.
Case 4: Appropriate vector of the lower lateral cartilages and partial right external nasal valve insufficiency. Surgery: correction of nasal deviation, smooth lowering of the dorsum, bilateral alar contour graft, and alar base reduction (Figure 13).
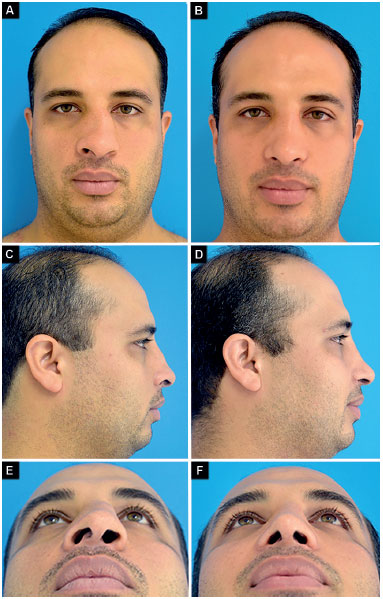 Figure 13.
Figure 13. Preoperative and postoperative period of 9 months.
A-B: Frontal;
C-D: Profile;
E-F: Deep inspiration.
DISCUSSION
The vector of the lower lateral cartilage is an important anatomical parameter that, in addition to defining the aesthetic characteristics of the tip and nasal alae, also points out existing functional problems related to external nasal valve insufficiency6-10,22.
Constantian9,10 classified the vector as appropriate when the straight line drawn between the domus and the site of insertion of this cartilage, in the pyriform aperture, matches or is lateral to the pupil. However, when this straight line is projected medially to the pupil, the vector is considered inappropriate.
Toriumi and Asher22 defined inappropriate vector when the angle formed by the lateral crus of the lower lateral cartilage with the sagittal plane is lower than 30º.
In our study, the vector was found inappropriate in 54% of the cases. This corroborated the results presented by Constantian9, who pointed out this change in 46% of the 100 consecutive rhinoplasties.
In another study, which included 200 consecutive rhinoplasties, the same author observed that this change appeared in 68% of primary rhinoplasties and 87% of secondary rhinoplasties performed10.
In our study, the relationship between the vector of the lower lateral cartilage and the external nasal valve insufficiency was statistically significant (p = 0.006) in the preoperative period. In 90% of the cases that presented an inappropriate vector, we observed external nasal valve insufficiency, in agreement with Constantian10, who diagnosed this condition in 100% of patients with an inappropriate vector of lower lateral cartilages.
This author highlighted that situations described as boxy tip and bulbous tip, in general, reflects the inappropriate positioning of lower lateral cartilages and the functional consequences previously described10.
To correct external nasal valve insufficiency, structuring medial and lateral walls is necessary. In our study, a columellar strut graft was used in all the cases for medial wall reinforcement, in accordance with the procedure described by Rohrich et al.19.
The lateral wall, represented by the nasal alae, was structured with grafts. These fully corrected external nasal valve insufficiency in 90% of the cases (p = 0,006), a result similar to that obtained by Rohrich et al.8, who were successful in 91% of patients treated with structuring of the nasal alae.
Given the importance of this subject, Constantian and Clardy4 studied septal and valve surgery in rhinoplasty and found, by preoperative and postoperative rhinomanometry, that patients with pure external nasal valve insufficiency, with valve reconstruction, had a 2.6 times increase in nasal airflow.
These authors broke a paradigm, emphasizing that in the group of patients with septal and valve problems, the greatest respiratory gain is provided by valve treatment, as the treatment of septal deformity in this group did not add much to the respiratory gain. The authors also demonstrated that the treatment of septal deviation and the reconstruction of internal and external nasal valves increased nasal airflow by 4.9 times4.
Today, purely reduction surgery is indicated in a small number of cases as demonstrated by Constantian9, who emphasized that only a low number of patients who underwent primary rhinoplasty (23%) benefited from the technique without developing further valve problems. In our study, nasal tip reduction surgery was performed in 32% of the cases and the columellar strut graft was always maintained. No valve complications were observed.
Changes in the lower lateral cartilages vector were reported for the first time in 1979, when Webster and Smith23 described the caudal repositioning of these cartilages without using grafts. Only in 1997, Gunter and Friedman6 described the lateral crural strut graft to reposition and/or strengthen lower lateral cartilages. In our study, this graft was used in 50% of the cases, which corrected the external valve insufficiency and improved nasal alae support6,22,25-28.
When the lower lateral cartilage has an inappropriate vector, the lateral wall of the external nasal valve lacks adequate support and is predisposed to conditions of valve insufficiency6,7,9,10,15,22,24. In this situation, the repositioning of the lower lateral cartilage will strengthen and provide a more harmonious nasal aspect, better forming the Anderson's tripod6,9,10,13,22,23,25-27.
Despite the appropriate vector, if lower lateral cartilages are weak and resection of the cranial portion of the lower lateral cartilages is needed from an aesthetical point of view, which would further weaken these cartilages, a preventive treatment for external nasal valve insufficiency is required9.
In the present study, preventive treatment with lateral crural strut graft was performed without changing the vector of these cartilages. The alar contour graft was also used, depending on the case.
The alar contour graft was described for the first time by Troell et al.29, who used costal cartilages irradiated for its creation. In our study, we followed the technique described by Rohrich et al.8, who used autologous cartilage to make this graft, which they performed in 100% of the cases8,20. However, in our series, the use of this graft was required only in 32% of the cases.
This agrees with the finding of Guyuron et al.21, who described the use of alar contour graft in 39% of 1,427 rhinoplasties performed. In a more recent series of 100 cases, the authors reported the use of this graft in 88% of the patients.
To create the pocket for this graft, Rohrich et al.8 used Stevens scissors. Conversely, it is easier for us to create the pocket with a 2 mm osteotome.
Gruber30 relates that when an alar contour graft wider than 3-4 mm is necessary, the choice then is the lateral crural strut graft. This was routinely used in our study and we verified functional improvement.
In cases presenting a narrow alar base, in which the lateral crural strut graft could cause a bulge in the nasal lining that would lead to obstructive external valve insufficiency, the alar contour graft was chosen, as pointed out by some authors20,22,26.
In the Southern region, owing to the ethnic characteristics of the population presenting a narrow alar base, the consensus is to use the above-mentioned procedure.
Regarding the frequency of the grafts used, this study agrees with most authors on the higher use of the grafts while performing secondary rather than primary rhinoplasty, which allows achieving adequate nasal function4,9,10,24,27,28.
CONCLUSION
The inappropriate vector of lower lateral cartilages resulted, in 74% of the cases, in an unstructured nasal alae and external nasal valve insufficiency. Structuring of the nasal alae with the lateral crural strut graft and/or alar contour graft proved to be effective in the correction of the external nasal valve insufficiency in 90% of the cases. In addition, it provides better aesthetic proportions and nasal contours. The frequency of grafts used in secondary rhinoplasty (80%) was greater than that in primary (66%), showing the need to use grafts in a more complex surgery.
COLLABORATIONS
ENS Analysis and/or interpretation of data; statistical analyses; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
RCB Analysis and/or interpretation of data; final approval of the manuscript; writing the manuscript or critical review of its contents.
REFERENCES
1. Howard BK, Rohrich RJ. Understanding the nasal airway: principles and practice. Plast Reconstr Surg. 2002;109(3):1128-44. DOI: http://dx.doi.org/10.1097/00006534-200203000-00054
2. Teichgraeber JF, Wainwright DJ. The treatment of nasal valve obstruction. Plast Reconstr Surg. 1994;93(6):1174-82. DOI: http://dx.doi.org/10.1097/00006534-199405000-00010
3. Constantian MB. The incompetent external nasal valve: pathophysiology and treatment in primary and secondary rhinoplasty. Plast Reconstr Surg. 1994;93(5):919-31. DOI: http://dx.doi.org/10.1097/00006534-199404001-00004
4. Constantian MB, Clardy RB. The relative importance of septal and nasal valvular surgery in correcting airway obstruction in primary and secondary rhinoplasty. Plast Reconstr Surg. 1996;98(1):38-54. DOI: http://dx.doi.org/10.1097/00006534-199607000-00007
5. Meyer R, Jovanovic B, Derder S. All about nasal valve collapse. Aesthetic Plast Surg. 1996;20(2):141-51. DOI: http://dx.doi.org/10.1007/BF02275534
6. Gunter JP, Friedman RM. Lateral crural strut graft: technique and clinical applications in rhinoplasty. Plast Reconstr Surg. 1997;99(4):943-52. DOI: http://dx.doi.org/10.1097/00006534-199704000-00001
7. Constantian MB. Four common anatomic variants that predispose to unfavorable rhinoplasty results: a study based on 150 consecutive secondary rhinoplasties. Plast Reconstr Surg. 2000;105(1):316-31. DOI: http://dx.doi.org/10.1097/00006534-200001000-00049
8. Rohrich RJ, Raniere J Jr, Ha RY. The alar contour graft: correction and prevention of alar rim deformities in rhinoplasty. Plast Reconstr Surg. 2002;109(7):2495-505. DOI: http://dx.doi.org/10.1097/00006534-200206000-00050
9. Constantian MB. The two essential elements for planning tip surgery in primary and secondary rhinoplasty: observations based on review of 100 consecutive patients. Plast Reconstr Surg. 2004;114(6):1571-81. DOI: http://dx.doi.org/10.1097/01.PRS.0000138755.00167.F5
10. Constantian MB. The boxy nasal tip, the ball tip, and alar cartilage malposition: variations on a theme--a study in 200 consecutive primary and secondary rhinoplasty patients. Plast Reconstr Surg. 2005;116(1):268-81. PMID: 15988278 DOI: http://dx.doi.org/10.1097/01.PRS.0000169958.83870.E1
11. Rhee JS, Arganbright JM, McMullin BT, Hannley M. Evidence supporting functional rhinoplasty or nasal valve repair: A 25-year systematic review. Otolaryngol Head Neck Surg. 2008;139(1):10-20. PMID: 18585555 DOI: http://dx.doi.org/10.1016/j.otohns.2008.02.007
12. Pereira MD, Marques AF, Ishida LC, Smialowski EB, Andrews JM. Total reconstruction of the alar cartilage en bloc using the ear cartilage: a study in cadavers. Plast Reconstr Surg. 1995;96(5):1045-52. DOI: http://dx.doi.org/10.1097/00006534-199510000-00006
13. Daniel RK. The nasal tip: anatomy and aesthetics. Plast Reconstr Surg. 1992;89(2):216-24. DOI: http://dx.doi.org/10.1097/00006534-199202000-00002
14. Sheen JH. Aesthetic rhinoplasty. 1st ed. St. Louis: Mosby, 1979. p. 432-62.
15. Hamra ST. Repositioning the lateral alar crus. Plast Reconstr Surg. 1993;92(7):1244-53. PMID: 8248399
16. Sheen JH, Constantian MB. Four common anatomic variants that predispose to unfavorable rhinoplasty results: a study based on 150 consecutive secondary rhinoplasties. Plast Reconstr Surg. 2000;105(1):332-31 [discussion]. DOI: http://dx.doi.org/10.1097/00006534-200001000-00050
17. Rohrich RJ, Hoxworth RE, Thornton JF, Pessa JE. The pyriform ligament. Plast Reconst Surg. 2008;121(1):277-81. PMID: 18176231 DOI: http://dx.doi.org/10.1097/01.prs.0000293880.38769.cc
18. Gunter JP, Landecker A, Cochran CS. Frequently used grafts in rhinoplasty: nomenclature and analysis. Plast Reconstr Surg. 2006;118(1):14e-29e. PMID: 16816668 DOI: http://dx.doi.org/10.1097/01.prs.0000221222.15451.fc
19. Rohrich RJ, Hoxworth RE, Kurkjian TJ. The role of the columellar strut in rhinoplasty: indications and rationale. Plast Reconstr Surg. 2012;129(1):118e-25e. DOI: http://dx.doi.org/10.1097/PRS.0b013e3182362b7a
20. Unger JG, Roostaeian J, Small KH, Pezeshk RA, Lee MR, Harris R, et al. Alar Contour Grafts in Rhinoplasty: A Safe and Reproducible Way to Refine Alar Contour Aesthetics. Plast Reconst Surg. 2016;137(1):52-61. DOI: http://dx.doi.org/10.1097/PRS.0000000000001942
21. Guyuron B, Bigdeli Y, Sajjadian A. Dynamics of the alar rim graft. Plast Reconstr Surg. 2015;135(4):981-6. PMID: 25502861 DOI: http://dx.doi.org/10.1097/PRS.0000000000001128
22. Toriumi DM, Asher SA. Lateral crural repositioning for treatment of cephalic malposition. Facial Plast Surg Clin North Am. 2015;23(1):55-71. DOI: http://dx.doi.org/10.1016/j.fsc.2014.09.004
23. Webster RC, Smith RC. Lateral crural retrodisplacement for superior rotation of the tip in rhinoplasty. Aesthetic Plast Surg. 1979;3(1):65-78. PMID: 24173975 DOI: http://dx.doi.org/10.1007/BF01577838
24. Gubisch W, Eichhorn-Sens J. Overresection of the lower lateral cartilages: a common conceptual mistake with functional and aesthetic consequences. Aesthetic Plast Surg. 2009;33(1):6-13. PMID: 19037690 DOI: http://dx.doi.org/10.1007/s00266-008-9267-y
25. Toriumi DM. New concepts in nasal tip contouring. Arch Facial Plast Surg. 2006;8(3):156-85. DOI: http://dx.doi.org/10.1001/archfaci.8.3.156
26. Mangaravite R, Bouchama A, Sinder R. Deformidade das cartilagens alares em forma de parênteses: tratamento pela rotação caudal das crura laterais, experiência de 49 casos. Rev Bras Cir Plást. 2014;29(1):50-6.
27. Pochat VD, Alonso N, Meneses JVL. Avaliação funcional e estética da rinoplastia com enxertos cartilaginosos. Rev Bras Cir Plást. 2010;25(2):260-70.
28. Almeida GS. Tratamento das válvulas nasais em rinoplastia secundária. Rev Bras Cir Plást. 2013;28(3):422-7.
29. Troell RJ, Powell NB, Riley RW, Li KK. Evaluation of a new procedure for nasal alar rim and valve collapse: nasal alar rim reconstruction. Otolaryngol Head Neck Surg. 2000;122(2):204-11. PMID: 10652391
30. Gruber RP, Rohrich RJ, Raniere J Jr, Ha RY. The alar contour graft: correction and prevention of alar rim deformities in rhinoplasty. Plast Reconstr Surg. 2002;109(7):2506-8 [discussion]. DOI: http://dx.doi.org/10.1097/00006534-200206000-00051
1. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
2. Faculdade Evangélica do Paraná, Curitiba, PR, Brazil
3. Universidade Estadual de Ponta Grossa, PR, Brazil
4. Hospital Universitário Cajuru da Pontifícia Universidade Católica do Paraná, Curitiba, PR, Brazil
Institution: Private clinic.
Corresponding author:
Eduardo Nascimento Silva
Avenida Doutor Francisco Búrzio, 991
Ponta Grossa, PR, Brazil Zip Code 84010-200
E-mail: dr_eduardosilva@yahoo.com.br
Article received: June 27, 2016.
Article accepted: September 19, 2016.
Conflicts of interest: none.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license