ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Cultural adaptation of rhytidectomy outcome evaluation questionnaire: facial outcome evaluation
Adaptação cultural do questionário de avaliação de resultados em ritidoplastia: facial outcome evaluation
ABSTRACT
INTRODUCTION: Quality of life questionnaires have been shown to be useful to confer greater objectivity to the evaluation of treatment outcomes. In turn, the internationalization of these instruments allows the comparison of different populations, although it requires a specific methodology to avoid misinterpretations due to translation errors or cultural differences. The Facial Outcome Evaluation (FOE) questionnaire, translated to English, is an instrument that is easy to apply and is based on objective questions; therefore, it is highly relevant for this purpose. The questionnaire has already been tested in relation to its reliability, validity, and responsiveness.
OBJECTIVES: To translate and culturally adapt to the Brazilian Portuguese version of the FOE questionnaire.
METHODS: The questionnaire was translated and culturally adapted to Portuguese, according to the methodology proposed by Beaton et al., based on the following 5 phases: phase 1, translation by two native Portuguese speakers; phase 2, generation of a synthetic version; phase 3, reverse translation by two native English speakers; phase 4, review by an evaluation committee; and phase 5, application to a population of 20 people.
RESULTS: The questionnaire was translated and successfully adapted, without comprehension problems in the final population.
Keywords:
Face; Rhytidectomy; Outcomes Evaluation; Questionnaire.
RESUMO
INTRODUÇÃO: O emprego de questionários de qualidade de vida (QV) tem se mostrado muito útil no sentido de dar maior objetividade à avaliação de resultados de tratamentos. A internacionalização desses instrumentos, por sua vez, permite a comparação interpopulacional, mas requer uma metodologia específica, a fim de não causar distorções devido a falhas na tradução ou a diferenças culturais. O questionário FOE (Facial Outcome Evaluation), de língua inglesa, é uma ferramenta de simples aplicação, com perguntas objetivas com boa aplicação para esse fim. O questionário já foi testado em relação à sua confiabilidade, validade e capacidade de resposta.
OBJETIVOS: Traduzir e adaptar culturalmente para o português brasileiro o questionário Facial Outcome Evaluation.
MÉTODOS: Realizada tradução e adaptação cultural para a língua portuguesa, segundo a metodologia proposta por Beaton et al., na qual existem 5 estágios. Estágio 1 - tradução por meio de dois tradutores nativos de língua portuguesa. Estágio 2 - confecção de versão de síntese. Estágio 3 - tradução reversa por dois tradutores nativos de língua inglesa. Estágio 4 - revisão por um comitê avaliador. Estágio 5 - aplicação a uma população de 20 pessoas.
RESULTADOS: O questionário foi traduzido e adaptado com sucesso, sem problemas de compreensão para a população final.
Palavras-chave:
Face; Ritidoplastia; Avaliação de resultados; Questionários.
INTRODUCTION
The search for a greater objectivity in the evaluation of treatment outcomes is necessary to improve the levels of evidence, especially in plastic surgery, as the aim is to achieve a subjective parameter (i.e., to improve the quality of life)1.
The literature clearly indicates that the mechanisms to measure objective outcomes of cosmetic procedures are still under development. However, these points toward the tendency of using instruments to measure outcomes, which, according to Morley, are reported by patients themselves (PROM or PRO) by means of questionnaires. Fortunately, major advances have been made already in this area, with the publication of several articles proposing questionnaire models2.
Breast-Q, Face-Q, and the Satisfaction with Facial Appearance Scale and Skindex tools, for instance, were already subjected to a rigorous process of validation and are fully in agreement with the requirements necessary for their acceptance by the US Food and Drug Administration. Along with the Skindex, these instruments stand out in relation to other PROMs according to Morley2.
Kosowski et al.3 found 442 articles on outcome evaluation referring to aesthetic, surgical, or non-surgical procedures. Of these, 47 were specific to facial appearance. However, only 9 satisfied the inclusion and exclusion criteria of their study. None of them entirely met the guidelines. All of the tools showed limitations due to their development, validation, or content. In this study, only the Face-lift Outcome Evaluation (FOE) showed to be a specific instrument for rhytidectomies.
FOE was described by Alsarraf4, along with other questionnaires in English that are specific for blepharoplasty (Blepharoplasty Outcome Evaluation [BOE]), rhinoplasty (Rhinoplasty Outcome Evaluation [ROE]), skin rejuvenation, and facial procedures (Skin Rejuvenation Outcome Evaluation [SROE]) questionnaires.
These questionnaires address physical, mental, and social aspects that are necessary to conduct a proper evaluation2. The FOE questionnaire was tested with regard to its validity, reliability, and responsiveness, and presented as a reliable quantitative instrument for the evaluation of the outcomes obtained2,5,6.
In turn, the internationalization of these questionnaires allows comparing treatment outcomes among different populations. However, care should be taken to avoid misinterpretation due to translation errors or cultural differences that may alter per se the result of the questions. This would reduce the comparative interpopulation value7,8.
Of the four questionnaires elaborated by Alsarraf, so far, only ROE was translated to Portuguese.
The others, namely BOE, FOE, and SROE, have still not been translated to Portuguese9.
FOE is composed of six questions, as shown in Annex 1. Each answer can be scored from 0 (the least satisfied possible) to 4 (the most satisfied possible). These values (marked) should be added, divided by 24 and multiplied by 100, in order to obtain a score from 0 to 100, with 0 representing the least satisfied possible and 100 the most satisfied possible4.
This instrument can be of great benefit to the development of scientific studies and outcome follow-up by surgeons.
Therefore, the objective of this study was to translate and culturally adapt the FOE questionnaire into Brazilian Portuguese.
MATERIALS AND METHODS
The study was approved by the research ethics committee of the Federal University of Ceará.
The FOE questionnaire was translated and culturally adapted to Brazilian Portuguese according to the methodology proposed by Beaton et al. and in agreement with the flowchart illustrated in Figure 1. This methodology consists of five phases and is accepted in the literature for the translation of several other instruments7.
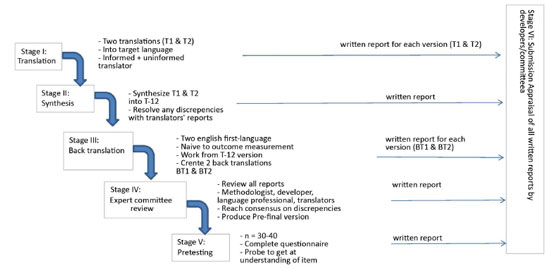
Figure 1. Flowchart representing the methodology of the translation and cultural adaptation proposed by Beaton el al.
7
Translation
Phase 1: The questionnaire underwent two different translations (T1 and T2), from English into Portuguese. One was conducted by a lay translator and the other was translated by a plastic surgeon with experience in the procedure, as recommended in the literature.
Phase 2 (Synthesis): T1 and T2 Portuguese versions of the questionnaires were evaluated by both translators of phase 1, who discussed the differences found in their versions and reached an agreement elaborating a consensus version, called T-12.
Phase 3 (Reverse translation): Questionnaire T-12 was reviewed by two lay translators who were blinded to one of the other and of the ongoing study. These were native English speakers.
Phase 4 (Submission to an expert committee): A medical board, expert in the area, was asked to monitor this process, evaluating these versions and pointing out inconsistencies and meaning deviations. The board was composed of a dermatologist, a general surgeon, and an orthopedist.
Upon discussions, a consensus was achieved on the following four points:
- Semantic Equivalence. The translations were evaluated with regard to the preservation of their meaning and the possibility of multiple interpretations and the existence of grammatical difficulties.
- Idiomatic Equivalence. Expressions or colloquialisms are difficult to translate. The committee sought the presence of these expressions and their equivalent in the Portuguese language.
- Experimental Equivalence. The experiences questioned were evaluated with regard to their existence in the Portuguese language.
- Conceptual Equivalence. The expressions must preserve the original concept. For instance, when talking about family, in some cultures, the meaning is of a small and direct family while others include all the relatives.
Cultural Adaptation
Phase 5 (Pre-final version of the test). A pretest with the final T-12 version was conducted with a sample of 20 people. This group was composed of graduate students of dermato functional physical therapy. Each participant completed the questionnaire and was interviewed by the researcher to identify possible inconsistencies and difficulties in understanding.
Phase 6 (Submission of the documents to an expert committee to verify the translation).
RESULTS
The FOE questionnaire was translated to the T1 and T2 versions. Several divergent points were found between the two versions. However, a consensus was reached and a T-12 final version was elaborated.
This version underwent two reverse translations, namely B1 and B2, which showed some divergences, although the original meaning was preserved.
The B1 and B2 versions were analyzed by the author of the original questionnaire, who was contacted by e-mail and who did not find any change in the meaning or inconsistency between the questionnaire translated from Portuguese to English and its original version.
No difficulties regarding the completion and understanding of the questionnaires were detected.
The committee evaluated every step of the translation and contributed suggestions for several changes that were subsequently carried out, as accepted. The final result of the translation is as shown in Annex 2.
DISCUSSION
No major difficulties were detected in the translation of the questionnaires, due to the small number of expressions without the correspondent translation to Portuguese.
We believe that quality-of-life questionnaires are important to render more objective and several comparable subjective parameters. This allows comparison of results and providing better levels of evidence in an area of knowledge that lacks these. However, no perfect questionnaire has been developed.
The comparison of different instruments is beyond the scope of this study. However, although the FOE questionnaire statistically showed good validity, reliability, and responsiveness, its design does not seem to be as well-grounded as that of a Face-Q questionnaire, which provides a significant interval level. This allows generating units, which are defined with a constant distance between each other. This means, for instance, that if a person's score is increased from 100 to 120, the increase is similar to that in the score of an individual from 120 to 140, contrarily to most of other instruments10.
On the other hand, unlike Face-Q, the FOE questionnaire is specific for rhytidectomy procedures and is freely available to everyone; these features facilitate its translation. The completion of the FOE questionnaire takes less than 1 minute.
The limitations of the FOE questionnaire are the absence of cutoff points and levels of normality, even in its original language, which we would like to suggest for future studies.
REFERENCES
1. Ferreira MC. Cirurgia Plástica Estética - Avaliação dos Resultados. Rev Soc Bras Cir Plast. 2000;15(1):56-66.
2. Morley D, Jenkinson C, Fitzpatrick R. A structured review of patient reported outcome measures used for cosmetic surgical procedures. Report to Department of Health [acesso 02 Fev 2015]. Disponível em: http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Elective%20Cardiac_012011.pdf
3. Kosowski TR, McCarthy C, Reavey PL, Scott AM, Wilkins EG, Cano SJ, et al. A systematic review of patient-reported outcome measures after facial cosmetic surgery and/or nonsurgical facial rejuvenation. Plast Reconstr Surg. 2009;123(6):1819-27. PMID: 19483584 DOI: http://dx.doi.org/10.1097/PRS.0b013e3181a3f361
4. Alsarraf R. Outcomes research in facial plastic surgery: a review and new directions. Aesthetic Plast Surg. 2000;24(3):192-7. DOI: http://dx.doi.org/10.1007/s002660010031
5. Alsarraf R, Larrabee WF Jr, Anderson S, Murakami CS, Johnson CM Jr. Measuring cosmetic facial plastic surgery outcomes: a pilot study. Arch Facial Plast Surg. 2001;3(3):198-201. DOI: http://dx.doi.org/10.1001/archfaci.3.3.198
6. Alsarraf R. Outcomes instruments in facial plastic surgery. Facial Plast Surg. 2002;18(2):77-86. DOI: http://dx.doi.org/10.1055/s-2002-32197
7. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000;25(24):3186-91. DOI: http://dx.doi.org/10.1097/00007632-200012150-00014
8. Ferreira LF. Tradução para a língua portuguesa, adaptação cultural e validação do Breast Evaluation Questionnaire. (Dissertação de mestrado em Ciências) - Universidade de São Paulo; 2009.
9. Izu SC, Kosugi EM, Brandão KV, Lopes AS, Garcia LB, Suguri VM, et al. Normal values for the Rhinoplasty Outcome Evaluation (ROE) questionnaire. Braz J Otorhinolaryngol. 2012;78(4):76-9. PMID: 22936141 DOI: http://dx.doi.org/10.1590/S1808-86942012000400015
10. Pusic AL, Klassen AF, Scott AM, Cano SJ. Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013;40(2):249-60. DOI: http://dx.doi.org/10.1016/j.cps.2012.12.001
Sociedade Brasileira de Cirurgia Plastica, Fortaleza, CE, Brazil
Institution: Clínica Eduardo Furlani, Fortaleza, CE, Brazil.
Corresponding author:
Eduardo Antonio Torres Furlani
Rua Barbosa de Freitas, 1990, Aldeota
Fortaleza, CE, Brazil Zip Code 60170-021
E-mail: eduardo@eduardofurlani.com.br
Article received June 08, 2014.
Article accepted February 08, 2015.

Annex 1. The original FOE questionnaire, in English. Source: Alsarraf R. Outcomes research in facial plastic surgery: a review and new directions. Source: Aesthetic Plast Surg. 2000;24(3):192-7.

Annex 2. Final version of the FOE questionnaire, translated and adapted to Brazilian Portuguese.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license










