ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Venous thromboemboembolism prevention protocol in plastic surgery: results in 2759 patients at the Ivo Pitanguy Institute
Protocolo de prevenção de tromboembolismo venoso em cirurgia plástica: resultados em 2759 pacientes no Instituto Ivo Pitanguy
ABSTRACT
Introduction: Pulmonary embolism is the most predictable cause of death in hospitalized patients, even more in surgical patients. 200.000 new cases occur annually, with sudden onset and generally leading to death in the first 2 hours. Preventing is most effective than treating stablished disease. This study aims to show the importance and safety of the venous thromboembolism prevention protocol.
Methods: We conducted a retrospective study in the period between May 2009 and May 2011 at The Ivo Pitanguy Institute, where 2759 patients underwent plastic surgery (aesthetic and reconstructive). All patients were assessed for predisposing and exposing risk factors for venous thromboembolism and the sum of those factors generated a score determining the prophylactic procedure to be adopted according to the protocol.
Results: There were three cases of venous thromboembolism (0.1%): one case of pulmonary embolism and two cases of deep venous thrombosis. Chemoprophylaxis with heparin was administered in the three patients according to the venous thromboembolism prevention protocol. Our rates remained below those found in the literature, with a statistically significant difference in total cases (p < 0.0001). There were 34 cases of hematoma (1.2%): 55.9% in patients submitted to pharmacological prophylaxis with heparin and 44,1% in patients who used sequential compression devices only. The total rates of hematoma also remained below those found in the literature with a statistically significant difference (p < 0,001).
Conclusion: The venous thromboembolism prevention protocol of the Ivo Pitanguy Institute proved to be important and safe, preventing the occurrence of venous thromboembolism cases with low rates of hematoma.
Keywords:
Venous thrombosis; Heparin; Surgery, plastic; Disease prevention; Venous thromboembolism; Protocols.
RESUMO
INTRODUÇÃO: A embolia pulmonar é a causa de morte mais previsível em pacientes hospitalizados, sendo isso ainda mais prevalente em pacientes cirúrgicos. 200.000 novos casos ocorrem anualmente, com início súbito e geralmente levando à morte nas primeiras 2 horas. Prevenir é, portanto, mais efetivo que tratar a doença estabelecida. Esse estudo objetiva demonstrar a importância e segurança do protocolo de prevenção do tromboembolismo venoso.
MÉTODOS: Conduzimos um estudo retrospectivo no período de maio de 2009 a maio de 2011, quando 2759 pacientes foram submetidos à cirurgia plástica no Instituto Ivo Pitanguy. Todos os pacientes foram submetidos ao protocolo de prevenção e avaliados quanto aos fatores de risco para tromboembolismo venoso. A soma desses fatores gerou um escore que determinou a conduta profilática a ser adotada.
RESULTADOS: Houve três casos de tromboembolismo venoso (0,1%), sendo 1 de TEP e 2 de TVP. A quimioprofilaxia com enoxaparina administrada aos 3 pacientes de acordo com o protocolo de prevenção. Nossas taxas permaneceram abaixo das encontradas na literatura, com diferença estatisticamente significativa nos numero total de casos (p < 0,0001). Houve 34 casos de hematoma (1,2%), sendo 55,9% em pacientes submetidos à quimioprofilaxia e 44,1% em pacientes que usaram apenas o dispositivo de compressão pneumática intermitente apenas. As taxas totais de hematoma também permaneceram abaixo das encontradas na literatura, também com diferença estatisticamente significativa (p < 0,001).
CONCLUSÃO: O protocolo de prevenção do tromboembolismo venoso do Instituto Ivo Pitanguy se provou seguro e importante na prevenção dos casos de TEV, com taxas de hematoma abaixo do descrito na literatura.
Palavras-chave:
Venous thrombosis; Heparin; Surgery plastic; Disease prevention; Venous thromboembolism; Protocols.
INTRODUCTION
Pulmonary embolism (PE) is the most predictable cause of death in hospitalized patients. Every year 200,000 new cases occur; onset is sudden, and the disease usually leads to death within the first 2 hours1. Prevention is therefore more effective than treating the established disease.
Surgery is an important factor in the genesis of VTE (venous thromboembolism), since it links several predisposing factors such as tissue trauma, position of the patient, hypovolemia, movement restriction, and blood stasis. The absolute risk of deep venous thrombosis (DVT) in the hospital environment is estimated at 10-20% for outpatients and 15-40% for surgical patients2. A study published in 2009 showed that 80% of plastic surgeons have experienced DVT and 53% have experienced PE3.
In 2001, Reinisch et al.4 reported DVT rates of 0.35% and PE rates of 0.14% in patients undergoing facelift surgery. Hughes5 identified that the risk of VTE is seven times greater when liposuction is associated with other surgeries. In 2003 Aly et al.6 found a 9.3% incidence of PE in patients undergoing circumferential dermolipectomy and 6.6% in abdominoplasty combined with other surgery.
Weinman & Salzman created a score for VTE risk factors in 1994, and defined a preventive approach through this classification7.
In 1999, the American Society of Plastic Surgery organized a task force suggesting some prophylactic measures for venous thromboembolism, but did not define a guideline8.
In 2003, a Brazilian study was published describing the protocol for VTE prevention developed in the department of plastic surgery at Israelita Albert Einstein Hospital, São Paulo. It was based on a multidisciplinary set up begun in 19999.
Rohrich & Rios10 and Davison et al.11 also developed a protocol including the routine use of low molecular weight heparin (LMWH).
In 2007 a protocol for venous thromboembolism prevention was established at the Ivo Pitanguy Institute12, based on the studies of Caprini et al.13 and Davison et al.11. In 2009, however, with the update of the ACCP guidelines (2008)14 and the publication of new specific studies in plastic surgery, this protocol was modified to add security, recommending earlier initiation of chemoprophylaxis with heparin in some cases.
There is a considerable resistance among surgeons to adoption of chemoprophylaxis because of the fear of increased bleeding events and resulting complications.
This paper demonstrates the importance of the prevention protocol for VTE established at the Ivo Pitanguy Institute and shows the safety of LMWH as a prophylactic measure in plastic surgery.
METHODS
A retrospective study was conducted between May 2009 and May 2011. During this period, 2759 patients underwent aesthetic and reconstructive plastic surgery at the Ivo Pitanguy Institute, which is composed of the Ivo Pitanguy Clinic and the 38th Infirmary of the General Hospital of Santa Casa de Misericórdia do Rio de Janeiro.
The prevention protocol for thromboembolic disease at the Ivo Pitanguy Institute was based on current guidelines of the American College of Chest Physicians (ACCP)14 combining the models of Patronella et al.15, Young & Watson16 and Anger et al.9, and is demonstrated in Appendix 1.
As detailed above, patients are evaluated in two steps generating a score that determines the prophylactic procedure to be adopted. The first step analyzes the exposing risk factors in association with clinical settings. A score is generated in this first step. The second step involves assessment of the predisposing risk factors associated with the patient. A score is produced again in this second step, and the sum of those two steps yields an overall score that classifies the patient in a risk group that determines the specific prophylactic recommendations. Patients are stratified into four risk groups: low risk (total sum of factors up to 2), moderate risk (3-4 factors), high risk (5-6 factors), and highest risk (> 6 factors). Chemoprophylaxis with heparin is started in patients in the moderate risk group 12 hours after the procedure. Heparin is indicated closer to the surgical event as the degree of risk increases (6 hours after surgery in the high risk group, and 1 hour before the surgery in the highest risk group). It is recommended that patients in the high risk and highest risk groups undergo a preoperative venous ultrasound exam of the lower limbs and to maintain LMWH for at least 2 to 5 days.
In large undermining surgeries, administration of half the dose of LMWH is considered, and in cases of spinal block administration of LMWH is started 12 hours after the procedure. The chemical prophylaxis adopted is a low molecular weight heparin such as enoxaparin 40 mg/day, continuing for 2 or more days, depending on the risk group. Early ambulation is encouraged, and all patients walk on the evening of the surgery. Elastic stockings are placed on the patient before the beginning of the procedure and maintained for 1 week in all patients. The intermittent pneumatic compression device is also used in all patients; it is turned on after anesthetic induction, and maintained until the next day. Patients are positioned on the operating table with the knees slightly flexed at five degrees in order to maximize blood flow.
The established criterion for defining hematoma was that described by the Subcommittee on Control of Anticoagulation of the International Society on Thrombosis and Homeostasis, which considers hematoma as a bleeding at the surgical site that needs a second intervention17.
The statistical analysis was performed with Fisher's exact test. We used the significance limit of 0.05, and declared a correlation significant if p-value < 0.05. All patients signed an informed consent that explained the current VTE prevention protocol before surgery. The research protocol was approved by the local Ethical Committee.
RESULTS
During the study period, 2759 patients were evaluated. The average age was 38.7 years, ranging from two years to 86 years. Single plastic surgery procedures comprised 2517 (91.2%) surgeries, and 242 (8.8%) were combined procedures (more than one plastic surgery procedure at the same time). Mammaplasty was the most prevalent surgery, accounting for 36.7% of the procedures. The second most prevalent surgery was abdominoplasty (14.6%), followed by facelift (13.0%), rhinoplasty (7.1%), liposuction (6.0%), blepharoplasty (4.5%), and other procedures (18.1%) including otoplasty, hair transplant, calf implants, palate reconstruction, and others.
According to our protocol, 34.7% of patients were classified in the low risk group, 56.6% as moderate risk, 8.3% as high-risk, and 0.4% as highest risk. There was a higher prevalence of patients in the moderate risk group for VTE, where chemoprophylaxis is already performed.
During the study period there were three cases of venous thromboembolism (0.1%) and 34 cases of hematoma (1.2%). The three patients who presented VTE had at least four predisposing or exposing risk factors for VTE and were classified in the highest, high, and moderate risk groups. LMWH was administered, according to the established prevention protocol: 1 hour before surgery, 6 hours after surgery, and 12 hours after surgery.
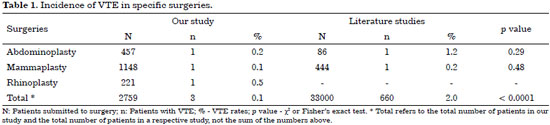
Table 1 shows the rates of VTE in specific plastic surgeries found in our study and in the literature.
Our VTE rates remained below those found in the literature, with a statistically significant difference in total cases (p < 0.0001).
Analyzing the 34 cases of hematoma, 19 (55.9%) took place after pharmacological prophylaxis with heparin, and 15 (44.1%) before the use of chemoprophylaxis.
Eighteen cases were observed after facelifts, 11 after mammaplasties, 2 after liposuctions, 2 after abdominoplasties, and 1 after blepharoplasty. According to prophylaxis used, 44.1% of the hematomas occurred in patients who did not use heparin, 44.1% in patients with heparin administered 12 hours after surgery, and 11.8% with heparin administered 6 hours after surgery. There were no cases of hematoma in the patients who received heparin 1 hour before surgery.
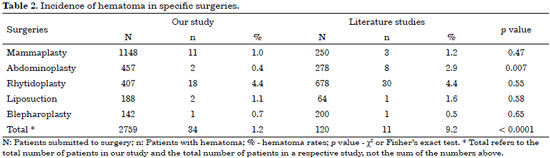
As can be seen in Table 2, the total rates of hematoma in our study remained below those found in the literature, with a statistically significant difference (p < 0.001). In abdominoplasties that difference was also statistically significant (p < 0.007). The rate of hematoma in blepharoplasty in our study was greater than the rate in the literature, but the difference was not statistically significant (p < 0.65).
DISCUSSION
At the Ivo Pitanguy Institute, all patients undergoing surgical procedures receive rigorous clinical evaluation by the Intensive Care Service. During this assessment, surgical risk is determined and the risk of VTE is identified, along with ideal prophylactic measures. The Intensive Care Service also provides intraoperative monitoring and rigid postoperative vigilance.
This protocol can be modified in exceptional conditions if bleeding complications arise during the course of the surgery, taking into account preoperative risk and the benefit of the pre-established measure.
In the first clinical evaluation prior to surgery, the patient is also instructed to discontinue medications with thrombogenic potential such as oral contraceptives and hormone replacement therapy. All patients are also encouraged to quit smoking. Since we have a large number of patients who come from other states and countries for surgery, they receive some recommendations long before surgery in order to minimize venous stasis during travel. Upon discharge, patients are instructed to drink plenty of fluids, walk several times a day, and use elastic stockings for seven days.
According to the recommendations in the 2008 ACCP guidelines14, drug prophylaxis should be initiated before or shortly after surgery, and should be continued until the patient is fully ambulatory.
In 2008 Patronella et al.15 conducted a retrospective study of 3871 patients and recommended LMWH 40 mg 1 hour after surgery, continuing for 3 days. Previously, Newall et al.18 demonstrated in 2006 that LMWH used 1 hour after surgery for 3 consecutive days in high-risk patients resulted in no cases of VTE without increasing rates of hematoma compared with the rates published in the literature.
In 2006 Young published recommendations for VTE prophylaxis in plastic surgery16. He divided patients into 4 risk groups; chemoprophylaxis with heparin was already administered in the moderate risk group, 2 hours before or 12 hours after surgery, and maintained until patient was fully ambulatory. In high and very high risk groups, this drug was administered in larger doses, 2 hours before or 12 hours after surgery, and maintained for 5 to 12 days.
In 2002 Anger et al.9 developed a protocol for VTE prevention directed at plastic surgery. In their protocol, heparin was recommended 2 or 12 hours before the surgical procedure according to the risk group.
There were many questions involving the elaboration of these VTE guidelines due to limited data in plastic surgery literature. What is the real incidence of VTE in plastic surgery? When is it the ideal time to start prophylaxis? What is the optimal dose?
The 2008 ACCP guidelines do not include plastic surgery, and there is a lack of category A or B evidence of established guidelines for thromboprophylaxis in plastic surgery.
VTE is the most important preventable cause of death after surgery. The preventive approach can only be conceived and established with the knowledge of the patient's risk conditions, which is the basic reason for preoperative evaluation. The current belief that VTE risk is low in plastic surgery patients is misleading. There is great difficulty in recognizing the disease, which can assume catastrophic proportions such as fatal pulmonary thromboembolism. Unfortunately, this may be the first and only manifestation of the disease19.
Heparin used close to surgery increased the effectiveness of prevention, and when heparin was used at half the usual dose in patients at high risk, 2 hours before or up to 4 to 6 hours after surgery, there was a reduction in DVT rates detected in venograph studies20. Delayed pharmacological prophylaxis leads to suboptimal antithrombotic effect without safety benefits21. There is no evidence of increased risk of hematoma with the use of heparin in plastic surgery, as demonstrated in the study published by Rohrich et al. in 200310. Metaanalysis and randomized double blind controlled trials showed little or no increase in the rate of bleeding with LMWH in general surgery2,22. In 2008, a study performed by Liao et al.23 concluded that there was no increased risk of hematoma after breast reconstruction with abdominal tissue with the use of chemoprophylaxis with heparin.
Some authors discuss the use of heparin in facelifts, where the prevalence of embolic events is presumably low, not worth the risk of bleeding events. We believe it is a great mistake to evaluate the patient's degree of VTE risk by only considering the type of plastic surgery performed. Each patient is unique, with a sum of characteristics that add up to greater or lesser risk. We also question the results of previous studies in plastic surgery of the incidence of VTE, given the low levels of evidence.
A review of 126 cases of rhytidectomies performed by the same surgeon showed a 5.6% incidence rate of hematoma requiring surgical revision, and a 16.2% rate of postoperative bleeding with prophylactic LMWH administered 2 hours before surgery24. The author reported the average length of surgery as 95 min (ranging from 45 to 145 minutes) from skin to skin.
Matarasso et al.25 in their study published in 2000 described a 4.4% incidence of hematomas after facelifts, without the use of LMWH.
In our study there were 3 cases of VTE and 34 cases of hematoma; the 3 VTE patients are described herein. The first case was a 50-year-old male undergoing nasal reconstruction due to neoplasia, who had a previous history of VTE. The second case was a 43-year-old female undergoing reconstructive mammaplasty after breast cancer. The last case was an abdominoplasty in a 35-year-old woman who was a smoker and did not walk after hospital discharge, remaining in bed most of the time. If we analyze the 3 cases, we realize that the patients who developed VTE had many factors predisposing them to this event. Two of them had malignant neoplasia, notoriously known as an important risk factor for VTE, as is previous history of VTE. The third patient did not respect the instructions to walk after discharge, and did not quit smoking prior to surgery as recommended. Immobility and smoking are also factors that increase the risk for VTE. All of these three patients received heparin according to the protocol, and used elastic stockings and the intermittent pneumatic compression device. The first patient presented pulmonary embolism and the others presented deep venous thrombosis. All three patients recovered and resumed their normal lives after the episode.
In the literature the rate of VTE in mammaplasties is stated as 0.23%26 and in abdominoplasties as 1.16%27. No data on the VTE rates in reconstructive rhinoplasty were found in the literature.
Our VTE rates remained below those found in the literature, with a statistically significant difference in total cases (p < 0.0001)3.
Among the 34 patients who presented hematomas, 19 cases (55.9%) were observed after chemoprophylaxis and 15 cases (44.1%) occurred without the administration of chemoprophylaxis. Patients receiving heparin 12 hours after surgery were the largest group. The group receiving heparin 1 hour before surgery presented no cases of hematoma. Epidemiological data from the 19 patients who presented hematoma after the administration of heparin showed that 13 of them had systemic arterial hypertension, 5 of them were dyslipidemic with regular use of statins, and 3 were taking vitamin E. It is well known that high blood pressure can damage vessels and promote bleeding. Statins are drugs that interfere with coagulation, leading to significant downregulation of the coagulation cascade, most probably as a result of decreased tissue factor expression, which leads to reduced thrombin generation28. Vitamin E is associated with hemorrhagic phenomena, acting as an anticoagulant by inhibiting platelet aggregation29.
The 34 cases of hematoma occurred in the following specific plastic surgeries: facelifts (18 cases), mammaplasties (11 cases), liposuctions (two cases), abdominoplasties (two cases), and blepharoplasty (one case).
Studies in the literature describing hematoma in plastic surgery indicated a 9.2% rate in the total number of plastic surgeries30 and in specific plastic surgeries as follows: 4.4% in facelift31, 2.9% in abdominoplasty32, 1.6% in lipoplasty33, 1.2% in mammaplasty34, and 0.5% in blepharoplasty35.
CONCLUSION
The Ivo Pitanguy Institute has always stood for excellence and pioneering in teaching, research, and healthcare practices in aesthetic and reconstructive plastic surgery. We must continuously strive to offer patients the best surgical outcome in the most secure conditions, which often involves changing habits and behaviors. Well-planned prevention with well-established criteria is the solution to avoid the high cost of exams, medical treatments, and preventable deaths. The prevention protocol we use at the Ivo Pitanguy Institute is safe and effective and does not increase the incidence of hematomas.
REFERENCES
1. King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: A metaanalysis. Chest. 2007;131(2):507-16.
2. Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):338S-400S.
3. Miszkiewicz K, Perreault I, Landes G, Harris PG, Sampalis JS, Dionyssopoulos A, et al. Venous thromboembolism in plastic surgery: incidence, current practice and recommendations. J Plast Reconstr Aesthet Surg. 2009;62(5):580-8.
4. Reinisch JF, Bresnick SD, Walker JW, Rosso RF. Deep venous thrombosis and pulmonary embolus after face lift: a study of incidence and prophylaxis. Plast Reconstr Surg. 2001;107(6):1570-5.
5. Jewell ML. Prevention of deep vein thrombosis in aesthetic surgery patients. Aesthet Surg J. 2001;21(2):161-3.
6. Aly AS, Cram AE, Chao M, Pang J, McKeon M. Belt lipectomy for circumferential truncal excess: the University of Iowa experience. Plast Reconstr Surg. 2003;111(1):398-413.
7. Weinman EE, Salzman EW. Deep-vein thrombosis. N Engl J Med. 1994;331(24):1630-41.
8. McDevitt NB. Deep vein thrombosis prophylaxis. American Society of Plastic and Reconstructive Surgeons. Plast Reconstr Surg. 1999;104(6):1923-8.
9. Anger J, Baruzzi ACA, Knobel E. Um protocolo de prevenção de trombose venosa profunda em cirurgia plástica. Rev Bras Cir Plást. 2003;18(1):47-54.
10. Rohrich RJ, Rios JL. Venous thromboembolism in cosmetic plastic surgery: maximizing patient safety. Plast Reconstr Surg. 2003;112(3):871-2.
11. Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114(3):43E-51E.
12. Paiva RA, Pitanguy I, Amorim NFG, Berger R, Shdick HA, Holanda TA. Tromboembolismo venoso em cirurgia plástica: protocolo de prevenção na Clínica Ivo Pitanguy. Rev Bras Cir Plást. 2010;25(4):583-8.
13. Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38(2 Suppl 5):12-9.
14. Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al.; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S-453S.
15. Patronella CK, Ruiz-Razura A, Newall G, Mentz HA, Arango ML, Assavapokee T, et al. Thromboembolism in high-risk aesthetic surgery: experience with 17 patients in a review of 3871 consecutive cases. Aesthet Surg J. 2008;28(6):648-55.
16. Young VL, Watson ME. The need for venous thromboembolism (VTE) prophylaxis in plastic surgery. Aesthet Surg J. 2006;26(2):157-75.
17. Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202-4.
18. Newall G, Ruiz-Razura A, Mentz HA, Patronella CK, Ibarra FR, Zarak A. A retrospective study on the use of a low-molecular-weight heparin for thromboembolism prophylaxis in large-volume liposuction and body contouring procedures. Aesthetic Plast Surg. 2006;30(1):86-95.
19. Hull RD, Brant RF, Pineo GF, Stein PD, Raskob GE, Valentine KA. Preoperative vs postoperative initiation of low-molecular-weight heparin prophylaxis against venous thromboembolism in patients undergoing elective hip replacement. Arch Intern Med. 1999;159(2):137-41.
20. Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, Soderberg K, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. The North American Fragmin Trial Investigators. Arch Intern Med. 2000;160(14):2199-207.
21. Hull RD, Pineo GF, Stein PD, Mah AF, MacIsaac SM, Dahl OE, et al. Timing of initial administration of low-molecular-weight heparin prophylaxis against deep vein thrombosis in patients following elective hip arthroplasty: a systematic review. Arch Intern Med. 2001;161(16):1952-60.
22. Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):287S-310S.
23. Liao EC, Taghinia AH, Nguyen LP, Yueh JH, May JW Jr, Orgill DP. Incidence of hematoma complication with heparin venous thrombosis prophylaxis after TRAM flap breast reconstruction. Plast Reconstr Surg. 2008;121(4):1101-7.
24. Durnig P, Jungwirth W. Low-molecular-weight heparin and postoperative bleeding in rhytidectomy. Plast Reconstr Surg. 2006;118(2):502-9.
25. Matarasso A, Elkwood A, Rankin M, Elkowitz M. National plastic surgery survey: face lift techniques and complications. Plast Reconstr Surg. 2000;106(5):1185-95.
26. Stevens WG, Gear AJ, Stoker DA, Hirsch EM, Cohen R, Spring M, et al. Outpatient reduction mammaplasty: an eleven-year experience. Aesthet Surg J. 2008;28(2):171-9.
27. van Uchelen JH, Werker PM, Kon M. Complications of abdominoplasty in 86 patients. Plast Reconstr Surg. 2001;107(7):1869-73.
28. Undas A, Brummel-Ziedins KE, Mann KG. Statins and blood coagulation. Arterioscler Thromb Vasc Biol. 2005;25(2):287-94.
29. Kim JE, Han M, Hanl KS, Kim HK. Vitamin E inhibition on platelet procoagulant activity: involvement of aminophospholipid translocase activity. Thromb Res. 2011;127(5):435-42.
30. Seruya M, Venturi ML, Iorio ML, Davison SP. Efficacy and safety of venous thromboembolism prophylaxis in highest risk plastic surgery patients. Plast Reconstr Surg. 2008;122(6):1701-8.
31. Jones BM, Grover R. Avoiding hematoma in cervicofacial rhytidectomy: a personal 8-year quest. Reviewing 910 patients. Plast Reconstr Surg. 2004;113(1):381-7.
32. Stewart KJ, Stewart DA, Coghlan B, Harrison DH, Jones BM, Waterhouse N. Complications of 278 consecutive abdominoplasties. J Plast Reconstr Aesthet Surg. 2006;59(11):1152-5.
33. Pereira LH, Sterodimas A. Composite body contouring. Aesthetic Plast Surg. 2009;33(4):616-24.
34. Lejour M. Vertical mammaplasty: early complications after 250 personal consecutive cases. Plast Reconstr Surg. 1999;104(3):764-70.
35. Patrocinio TG, Loredo BA, Arevalo CE, Patrocinio LG, Patrocinio JA. Complications in blepharoplasty: how to avoid and manage them. Braz J Otorhinolaryngol. 2011;77(3):322-7.
1. Instituto Ivo Pitanguy, Rio de Janeiro, RJ, Brazil
2. Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Institution: Instituto Ivo Pitanguy, Rio de Janeiro, RJ, Brazil.
Corresponding author:
Rita Azevedo de Paiva
Rua Visconde Pirajá, 330 - Ipanema
Rio de Janeiro, RJ, Brazil Zip code 22410-001
E-mail: ritaazevedodepaiva@hotmail.com
Article received: September 30, 2014.
Article accepted: March 15, 2015.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license