ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Pyoderma gangrenosum after quadrantectomy with intraoperative radiotherapy: a case report
Pioderma gangrenoso após quadrantectomia de mama com radioterapia intraoperatória: relato de caso
Case Report -
Year2015 -
Volume30 -
Issue
1
Alfonso Sempértegui; Ismael Santiago Vasquez Vasquez; Antonio Luiz Rocha Gesualdi Fernandes Neto; Leandro Menezes Lopes dos Santos; Bruno César Freitas Alvarenga; Winston Joseph Ueda; Jose Tadeu Campos Avelar; Ernane Bronzatt; Liliana Maria de Oliveira Moscardini; Gabriela Ramos Alves; Henrique Moraes Salvador Silva
http://www.dx.doi.org/10.5935/2177-1235.2015RBCP0130
ABSTRACT
Pyoderma gangrenosum is an immune-mediated inflammatory and rare skin disease with an extremely challenging diagnosis. The clinical evolution of the disease is the basis for the diagnosis that involves pustular superficial lesions, painful erythematous halo, rapid progression to painful and sterile ulcerations, unresponsiveness to antibiotics or new surgical interventions, and finally, ready improvement with the use of immunosuppressive drugs. Delayed diagnosis may cause numerous hospitalizations and prolonged therapy, whereas early recognition can prevent the progression of the ulcerations and their morbidities. We report a case of pyoderma gangrenosum that evolved after surgery and was associated with intraoperative radiotherapy for the conservative treatment of breast cancer. In addition, we reviewed reported cases in the literature and therapeutic options. It is conjectured that intraoperative radiotherapy might be related to some immune-mediated stimuli that could trigger the clinical condition.
Keywords:
Pyoderma gangrenosum; Breast cancer; Intraoperative radiotherapy.
RESUMO
Pioderma gangrenoso é uma doença inflamatória imunomediada e rara da pele, de diagnóstico extremamente desafiador. A evolução clínica é a base para o diagnóstico, cursando com lesões pustulosas superficiais, halo eritematoso doloroso, rápida progressão para ulcerações dolorosas e estéreis, sem resposta a antibióticos ou a novas intervenções cirúrgicas e, finalmente, com pronta melhora com uso de imunossupressores. O atraso no diagnóstico pode acarretar numerosas internações e terapias prolongadas, sendo que seu reconhecimento precoce, por outro lado, evita a progressão dessas ulcerações e sua morbidade. Relatou-se um caso de pioderma gangrenoso que evoluiu após cirurgia associada à radioterapia intraoperatória no tratamento conservador do câncer de mama, fazendo-se uma revisão de casos relatados na literatura e suas possibilidades terapêuticas. Questiona-se, também, se a radioterapia intraoperatória estaria relacionada com algum estímulo imunomediado, o que poderia ter facilitado o desencadeamento do quadro.
Palavras-chave:
Pioderma gangrenoso; Câncer de mama; Radioterapia intraoperatória.
INTRODUCTION
The primary purpose of this case report is to present epidemiological knowledge on the occurrence of pyoderma gangrenosum (PG) after conservative surgery for breast cancer that is associated with intraoperative radiotherapy (IORT), in addition to a review of reported cases in the literature and existing therapeutic options. PG is a rare and extremely challenging condition for the treatment team.
CASE REPORT
A female patient aged 51 years had been previously healthy at the routine breast cancer examination and presented no changes in physical examination findings. However, mammographic finding showed a 1.2-cm partially defined nodule in the superolateral right quadrant (SRQ) and absence of microcalcifications.
On ultrasonographic examination, a uniform nodule with irregular borders (12 × 9 × 8 mm in size), located 5 cm from the nipple and 15 mm from the skin, was confirmed on Doppler flow imaging. Core Biopsy was performed and revealed invasive ductal carcinoma of the breast of grade III Bloom-Richardson (BR). On nuclear magnetic resonance imaging, other foci were not observed.
The patient was informed of the possible therapeutic options suitable for her case, and the risks and benefits of all surgical options. After consultation with a radiotherapist and as she filled the indications for IORT, the patient opted and underwent this treatment according to the standard protocol of the mastology team of Mater Dei Hospital. Sentinel lymph node biopsy and quadrantectomy with right IORT were planned.
The surgery proceeded without anomalies, extending for 1 h 30 min (skin to skin). After confirming the negative diagnosis of axillary sentinel lymph node macrometastases by performing an imprint cytological analysis, the patient underwent quadrantectomy in the SRQ of the right breast, followed by IORT. Radiotherapy was performed under the following conditions: time of exposure, 4 min 28 sec; a 6-cm cone in the breast parenchyma; depth of 3.5 cm; 15 MeV; volume of 98.9 cm3; and 21-Gy Boost. The patient had good progress in the immediate postoperative period.
The patient was discharged on the first postoperative day (POD), with only mild hyperemia in the surgical and irradiated sites. The pathological examination diagnosed invasive ductal carcinoma, grade III BR, 1.5 cm, estrogen and progesterone receptor negative, and Cerb-B2 positive. Immunohistochemistry assessment confirmed the sentinel lymph nodes negative for macrometastasis and micrometastases.
However, on the second POD, she began to feel intense pain; had edema in the right breast, which affected the right hemithorax to the ipsilateral scapula; had no signs of inflammation or any other alteration, and was afebrile. She was treated with anti-inflammatory medication and thereby achieved partial improvement.
On the third POD, she complained of intense fatigue and chills but was continually afebrile, and her surgical wound had good appearance. Administration of cephalexin 500 mg four times a day was instituted. She evolved with fever on the fourth POD with 38 °C, tachycardia, and spontaneous drainage of secretions through the surgical wound, which had a brown aspect, was odorless, and had ulceration in the surgical margins, with hyperemia affecting the entire right hemithorax (Figure 1).

Figure 1. Aspect of the surgical quadrantectomy wound at the 3rd postoperative day.
The patient was readmitted, presenting only leukocytosis (13,000/93% segmented). She was taken to the surgical room, and the plastic surgeon performed debridement of nonviable tissue and necrotic skin, drainage of a large volume of liquid with a brown aspect, with an aliquot sent for culture examination. A skin biopsy was performed and sent for pathological analysis, in addition to a culture examination. This was followed by thorough washing of the wound and primary closure with placement of a vacuum drain. In the immediate postoperative period, some improvement of the local hyperemia was observed.
During this time, our hypothesis was radionecrosis associated with infection of the surgical site.
After an infectologist evaluated the history and clinical picture of the patient, the antibiotic therapy was changed to ampicillin associated with sulbactam. The pathological examination of the biopsy revealed a skin fragment, with ulceration and tissue necrosis, and intense acute inflammatory and suppurative reactions that reached the subcutaneous tissue. Skin culture, as well as liquid culture, including assessment for Mycobacterium, was negative for bacteria.
With stable evolution, she was discharged from the hospital after 48 hours of hospitalization (second surgery), with the vacuum drain and antibiotic therapy maintained. The drain was removed after 4 days, with daily changing of dressing.
The surgical wound progressed to a growing and progressive tissue distress, and treatment with hyperbaric oxygen therapy (HBOT) was initiated, maintaining the antibiotics. Then, the patient was taken back to the operating room (19 days after the first surgery) for another wound debridement (Figure 2).
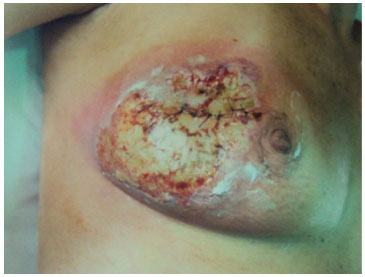
Figure 2. The surgical wound on the 19th postoperative day immediately before being subjected to the second surgical debridement.
On the 20th POD (first surgery), after discussing the past and present clinical framework and reviewing the complete medical history of the patient, the hypothesis of PG was proposed, with a view of progressive clinical worsening and laboratory tests without signs of infection (Figure 3).

Figure 3. Aspect of the wound on the 20th postoperative day, after the second surgical debridement and before the beginning of chemotherapy.
A meeting was convened with the Mastology, Plastic Surgery, Radiotherapy, Oncology, Infectious Disease, and General Medical teams, with the presence of the patient and her spouse.
Administration of corticosteroids 60 mg/day was initiated. At 22 POD (first surgery), after 24 hours of corticotherapy, the patient showed significant improvement in pain and onset of evolutionary granulation in the surgical wound. HBOT and daily dressing were maintained, and the antibiotics were suspended.
At 28 POD (first surgery), she received the first dose of infliximab. HBOT and corticosteroids were maintained until the 35 POD (first surgery), with gradual reduction of the dose due to improvement.
Chemotherapy was started at 60 POD (first surgery; doxorubicin hydrochloride + cyclophosphamide [4 cycles], followed by taxol [12 cycles]), which was well tolerated. With strict monitoring, the surgical wound displayed good evolution (Figure 4).
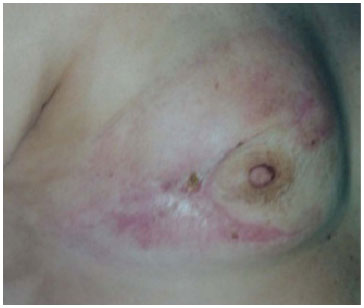
Figure 4. Wound showing good progress on the 60th postoperative day, after 40 days of chemotherapy and daily dressing.
DISCUSSION
Conservative treatment of breast cancer requires complementary radiotherapy (CXR) for the whole breast. This therapy has demonstrated its therapeutic value in the first therapy proposals based on the principles of Halsted. Currently, it plays an important role in conservative treatment, in locally advanced tumors and in the palliative treatment of metastases.
The recommended treatment of early-stage breast cancer consists of conservative surgery, followed by postoperative whole-breast radiotherapy. Current studies show that local recurrences after conservative breast surgery occur mainly in the quadrant of the primary tumor, suggesting that whole-breast radiotherapy may not be necessary. At the end of the 1990s, some studies of accelerated and partial breast irradiation, including brachytherapy, external CXR, and intraoperative CXR, were published. However, only a few of these studies compared between the technical applications1. The basis for the use of partial breast radiotherapy, instead of irradiating the whole breast, is based on the fact that 85% of all tumors recur next to the primary tumor bed. The main objective of IORT is to kill malignant cells in the operated area. IORT of the breast quadrant is performed after the removal of the primary tumor and can, in principle and in selected cases, replace the postoperative course of radiotherapy, which is currently used after conservative breast cancer surgery. With IORT being a new technique, no completed data from phase III trials have been published, although IORT is a promising treatment option for patients with early-stage breast cancer.
The European Institute of Oncology (Milan, Italy), the first center to implement the use of IORT in breast cancer, used a mobile linear accelerator to administer IORT. Based on assessments, the approved dose was 21 Gy, which had no important toxic effects, of which 10% are mild local reactions (redness, edema, pain, or infection)2.
After a mean follow-up of 20 months, the incidence of relapse (within the radiation field) in 590 women was 0.5%, with acute toxicity and acceptable cosmetic effects. One of the main benefits of IORT is the dramatic reduction in the total treatment time.
The radiotherapy wing of Mater Dei Hospital has been using this technique since 2007 for the treatment of breast cancer. Thus far, 45 cases of successful IORT have already been performed.
In accordance with the protocol of the service, the patient selection criteria are as follows: menopausal or perimenopausal patients aged 45 years or older; tumor size of up to 2.5 cm in imaging examination; sentinel lymph node biopsy negative for metastases in intraoperative freezing examination; invasive ductal carcinoma, with tubular or ductal infiltration in situ; and free margins of at least 5 mm.
Because it is a new procedure, definitive conclusions regarding the level of toxicity in the breast and other organs cannot be drawn. According to Veronessi, within a follow-up period of 24 months, early local toxicity was only 6% (mild fibrosis, liponecrosis, hematoma, and severe fibrosis)3. Late complications, occurring after 48 months of follow-up, include fat necrosis, fibrosis, and nodulations, which require complementary diagnostic assessment.
PG is an uncommon inflammatory skin disease, with an extremely challenging diagnosis. Originally described by Brocq in 1916, its occurrence after surgical procedures, however, was reported only in 1924 by Cullen4.
According to current literature, the highest incidence occurred in females, but PG can affect people of all ages. The occurrence in the breast is unusual5. Its association with other diseases (mainly autoimmune diseases) has been reported. It has also been reported as a severe complication after surgical procedures (pathergy lesions), evolving with devastating skin loss in postsurgical procedures6. The clinical evolution of the disease is the basis for diagnosis, coursing through superficial pustular lesions, painful erythematous halo, rapid progression to painful and sterile ulcerations, unresponsiveness to antibiotics or new surgical interventions, and finally, ready improvement with the use of immunosuppressants7.
Delayed diagnosis may cause numerous hospitalizations and prolonged therapy.
Meanwhile, early diagnosis prevents the progression of these ulcerations and their morbidity8.
Differential diagnosis of possible bacterial abscesses (always excluding infectious causes), gangrene after vascular thrombosis, necrotizing vasculitis, dermatitis, and necrotizing fasciitis should be made.
The initial treatment involves immunosuppressants. Different methods have been reported with success, including the use of systemic corticoids, dapsone, sulfapyridine, sulfasalazine, cyclophosphamide, and recently, topic tacrolimus. The first-line treatment is high-dose methylprednisolone (60-120 mg/day). If there is no improvement, cyclosporine is given as a second option9.
Another treatment option, also used in this case, consists of infliximab, a biological drug approved in several countries for use as treatment of autoimmune diseases. The drug is classified as a TNF-α inhibitor and has shown efficacy in PG cases associated with inflammatory bowel disease. Additional histological studies provide evidence that this inhibitor reduces the infiltration of inflammatory cells in affected areas. Its adverse reactions such as dyspnea, urticarial, and headache, which are the most common causes of treatment interruption, are related with its infusion.
HOBT is a therapeutic modality that consists of pure oxygen supplementation (FiO2= 100%) in a pressurized environment, at one level above atmospheric pressure. The indications for its use are as follows: embolism or gaseous gangrene; decompressive disease; Fournier syndrome; necrotizing infections of soft parts; radiation damage; acute anemia; acute traumatic ischemic, thermal, or electrical burns; refractory lesions; osteomyelitis; and compromised flaps or grafts. HOBT is a safe modality, presenting few contraindications. Its side effects are related to the pressure variation and/or oxygen toxicity. It has been considered in the treatment of PG, although its mechanism of action and effectiveness are not well established10.
Surgical treatment of PG should be avoided because of the risk of deterioration. However, in selected cases where the disease status is stabilized or declining, it may be necessary to remove necrotic tissue to prevent bacterial infection. Given the seriousness and the rapid developments in the case discussed herein, and after a ready response to therapy with corticosteroids, we finalized the diagnosis of PG.
In the treatment of PG in the present case, prednisolone 60 mg/day was used, with gradual dose reduction and infusions of infliximab, associated with 35 sessions of HOBT. The result was satisfactory. Unfortunately, the treatment of PG is still empirical owing to the lack of randomized controlled trials.
This case report aims to present epidemiological knowledge of this uncommon pathology, in addition to a review of reported cases in the literature and therapeutic options. Surgeons should consider PG among the differential diagnoses of postoperative complications, mainly because the sooner the treatment of PG is started, the greater the possibility of preventing important and unnecessary morbidities that may lead to patient death if untreated. Another issue that remains is whether radiotherapy is related to some immune-mediated stimuli that could trigger PG.
REFERENCES
1. Bernier J, Viale G, Orecchia R, Ballardini B, Richetti A, Bronz L, et al. Partial irradiation of the breast: old challenges, new solutions. Breast. 2006;15(4):466-75. http://dx.doi.org/10.1016/j.breast.2005.11.012. PMid:16439129.
2. Veronesi U, Orecchia R, Luini A, Gatti G, Intra M, Zurrida S, et al. A preliminary report of intraoperative radiotherapy (IORT) in limited-stage breast cancers that are conservatively treated. Eur J Cancer. 2001;37(17):2178-83. http://dx.doi.org/10.1016/S0959-8049(01)00285-4. PMid:11677104.
3. Veronesi U, Orecchia R, Luini A, Galimberti V, Gatti G, Intra M, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery: experience with 590 cases. Ann Surg. 2005;242(1):101-6. http://dx.doi.org/10.1097/01.sla.0000167927.82353.bc. PMid:15973107.
4. Cullen TS. A progressively enlarging ulcer of the abdominal wall involving the skin and fat, following drainage of an abdominal abscess apparently of appendiceal origin. Surg Gynecol Obstet. 1924;38:579-82.
5. Mansur AT, Balaban D, Göktay F, Takmaz S. Pyoderma gangrenosum on the breast: a case presentation and review of the published work. J Dermatol. 2010;37(1):107-10. http://dx.doi.org/10.1111/j.1346-8138.2009.00756.x. PMid:20175832.
6. Meyer TN. Pyoderma gangrenosum: a severe and ill-known complication of healing. Rev. Soc. Bras. Cir. Plást. 2006;21(2):120-4.
7. Schöfer H, Baur S. Successful treatment of postoperative pyoderma gangrenosum with cyclosporin. J Eur Acad Dermatol Venereol. 2002;16(2):148-51. http://dx.doi.org/10.1046/j.1468-3083.2002.00387.x. PMid:12046819.
8. Davis MD, Alexander JL, Prawer SE. Pyoderma gangrenosum of the breasts precipitated by breast surgery. J Am Acad Dermatol. 2006;55(2):317-20. http://dx.doi.org/10.1016/j.jaad.2006.02.066. PMid:16844520.
9. Friedman S, Marion JF, Scherl E, Rubin PH, Present DH. Intravenous cyclosporine in refractory pyoderma gangrenosum complicating inflammatory bowel disease. Inflamm Bowel Dis. 2001;7(1):1-7. http://dx.doi.org/10.1097/00054725-200102000-00001. PMid:11233655.
10. Rodrigues MJ, Marra AR. Quando indicar a oxigenoterapia hiperbárica? Rev Assoc Med Bras. 2004;50(3):229-51.
Hospital Mater Dei, Belo Horizonte, MG, Brazil
Institution: Study carried out at Hospital Mater Dei, Belo Horizonte, MG, Brazil.
Corresponding author:
Alfonso Sempértegui
Hospital Mater Dei
Avenida Raja Gabaglia, 1000, 5th floor - Cidade Jardim
Belo Horizonte, SP, Brazil Zip Code 30441-070
E-mail: alfonsosempertegui@yahoo.com.br
Article received: September 6, 2011.
Article accepted: January 28, 2012.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license











