ABSTRACT
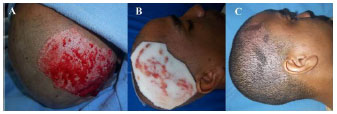
INTRODUCTION: The success of a skin graft is evaluated by not only the integration of the graft itself, but also the quality of the recovery of the donor site. Despite the fact that graft donor sites represent the best place to study wound healing, treatment regimens for donor sites have not been studied extensively.
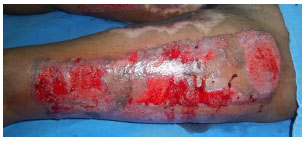
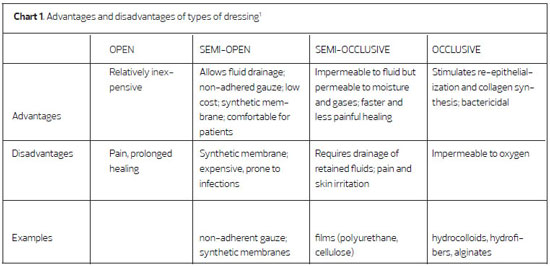
METHOD: To evaluate the efficiency of polyurethane foam as a dressing for graft donor sites. Methods: We conducted a prospective study in which graft donor sites were treated with polyurethane foam dressing, as an alternative to a cellulose acetate film.
RESULTS: We treated 11 patients and 14 donor graft sites. Problems associated with the use of polyurethane foam included prolonged adherence (73%) and an unpleasant odor (45%). The majority of patients reported that they found the dressing to be unsatisfactory (73%).
CONCLUSIONS: The use of a polyurethane foam was shown to be ineffective as a graft donor site dressing, due to the high rate of associated complications.
Keywords: Bandages; Dressing materials; Skin healing; Skin grafts; Donor site; Epithelialization