ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Histological and wall thickness assessment of organic capsules formed around smooth and textured tissue expanders in humans
Avaliação histológica e de espessura das cápsulas orgânicas formadas ao redor de expansores de tecidos de superfície lisa ou texturizada em humanos
Original Article -
Year2012 -
Volume27 -
Issue
2
João Medeiros Tavares Filho1; Tulia Cuzzi2; Diogo Franco3; Ivan Demolirani4; Talita Franco5
ABSTRACT
BACKGROUND: The search for an expander that can achieve tissue expansion, with minimum tissue damage, more distensible and thinner flaps, and fewer clinical side effects during the expansion process, has led to the testing of expanders with different surfaces textures (e.g., smooth vs. textured). The individual advantages of smooth and rough expanders are currently not known. This question has motivated research on capsules to determine which type of expander is best.
METHODS: We conducted a double-blind, prospective study with no exclusion criteria on 38 patients already undergoing tissue expansion at Plastic Surgery Department at the Universidade Federal do Rio de Janeiro (University Hospital of the Federal University of Rio de Janeiro). At the end of the expansion process, samples were collected from the edge, base, and dome of capsules formed around 28 smooth and 14 textured expanders. Differences in capsule wall thickness and histology were examined.
RESULTS: There was no difference in the clinical appearance, expansion time, or insertion planes between the two types of expanders. Additionally, no significant differences in histological characteristics were observed between the types of expander surfaces. In statistical analyses, no correlation between capsule wall thickness and expansion time or between the maximum and minimum wall thickness were observed.
CONCLUSIONS: Therefore, we conclude that the two types of tissue expander surfaces are equivalent.
Keywords:
Tissue expansion/methods. Tissue expansion/instrumentation. Tissue expansion devices.
RESUMO
INTRODUÇÃO: A busca por um produto que realize expansão tecidual com dano mínimo aos tecidos envolvidos, retalhos mais distensíveis e de menor espessura, e menos sintomas clínicos durante o processo de expansão tem levado as empresas a fabricar expansores com diferentes superfícies (lisa ou texturizada). A literatura é bastante controversa em relação às vantagens da utilização de um ou outro tipo. Esses aspectos motivaram os autores a pesquisar eventuais diferenças capsulares que pudessem justificar a escolha clínica.
MÉTODO: Foi realizado um estudo cego, prospectivo, sem critérios de exclusão, em 38 pacientes submetidos a expansão tecidual no Serviço de Cirurgia Plástica do Hospital Universitário da Universidade Federal do Rio de Janeiro (Rio de Janeiro, RJ, Brasil). Ao final do processo de expansão, foram coletados fragmentos da periferia, da base e da cúpula das cápsulas de 28 expansores de superfície lisa e de 14 de superfície texturizada, para estudo da espessura e de possíveis diferenças histológicas.
RESULTADOS: Não houve diferenças entre os dois tipos de expansor quanto aos aspectos clínicos, ao tempo efetivo de expansão e aos planos de inclusão. Na avaliação dos parâmetros histológicos, não foram observados valores significativamente diferentes segundo a superfície do expansor. Não houve correlação entre espessura e tempo de permanência, e a diferença entre as espessuras máxima e mínima foi considerada igual para os dois tipos de superfície.
CONCLUSÕES: Não há diferenças significativas do ponto de vista histológico, nem razões clínicas, que indiquem vantagens de um ou outro tipo de expansor no processo de expansão tecidual.
Palavras-chave:
Expansão de tecido/métodos. Expansão de tecido/instrumentação. Dispositivos para expansão de tecidos.
INTRODUCTION
The search for a tissue expander that achieves the desired expansion with minimum tissue damage has led to the testing of expanders with varying surface textures (e.g., smooth vs. rough). It is believed that some textures may reduce inflammation, leading to more distensible and thinner flaps with fewer clinical side effects during the expansion process.
It is currently not known which type of expander is best. Adams et al.1 have commented on the contradictory findings of various comparative studies on capsules formed around smooth and textured expanders. There have also been numerous reports on alterations in expanded skin, but few on variations in capsule histology. Of these, most have been performed on animals2-10. These issues motivated us to investigate the differences in capsule formation around the two types of expander surfaces. We hope that our results will lead to a better understanding of which type of capsule may be better histologically and for the patient as a whole.
The organic capsules that form around silicone implants continue to be studied for assessing their structure. It is hoped that a better understanding of their formation will allow prevention of pathological alterations. However, the study of these capsules in humans is usually limited to cases with complications that require implant removal. The use of tissue expanders provides the opportunity for observing early stage capsule formation at the time of removal which, on average, is about two months after insertion.
We conducted a double-blinded, prospective study in patients already undergoing tissue expansion for various indications and body locations. At the end of each patient's expansion process, we collected samples from the organic capsule that had formed around the tissue expander. Both smooth and textured expanders were studied so that differences in capsule histology and thickness could be determined. We also analyzed several variables such as anatomical region, expander insertion plane, expansion time, patient age, underlying anatomic buttress (i.e., rigid underlying structure [bone] vs. flexible structure [muscle]), and primary expansion vs. re-expansion.
METHOD
Thirty-eight patients underwent tissue expansion with smooth silicone expanders in 24 cases and textured expanders in 14 cases (Silimed, Rio de Janeiro, Brazil). Of the patients receiving smooth expanders, 16 were female and 8 were male, with ages ranging from 10 to 49 years. Of the patients receiving textured expanders, 13 were female and 1 was male, with ages ranging from 10 to 39 years. Expanders were placed in the subcutaneous plane in 28 cases, in the subfascial plane in 7 cases, and in the submuscular plane in 3 cases. Expanders were placed in the following locations: thigh (n = 10), abdomen (n = 7), scalp (n = 4), chest (n = 4), shoulder (n = 3), upper arm (n = 3), forearm (n = 3), leg (n = 2), face (n = 1), and the cervical region (n = 1). Indications for tissue expansion included post-burn scarring (n = 23), post-traumatic scarring (n = 8), post-mastectomy breast implant preparation (n = 3), hemangioma removal (n = 2), nevus removal (n = 1), and scleroderma sequelae (n = 1). Primary expansion was performed in 29 cases; smooth expanders were used in 16 and textured expanders in 13. Re-expansion was performed in 9 cases, 7 of which were first re-expansions and 2 were second re-expansions. Expansion time varied between 9 and 34 weeks for smooth expanders and between 9 and 25 weeks for textured ones.
The expansion process occurred according to standard procedures adopted by the Department of Plastic Surgery at the Hospital of the Federal University in Rio de Janeiro (UFRJ)11-13. At the end of the expansion process, the expander was removed and 1 cm × 3 cm capsule fragments were collected from three areas: the capsule edge, the base center, and the dome center (Figure 1). The fragments were then placed on sterilized filter paper (with the deep edge of the specimen in contact with the paper) and immersed in a 10% formaldehyde solution. Tissues were embedded in paraffin blocks, which were then sectioned into 4-μm histological slices. Sections were stained using hematoxylin and eosin stain.
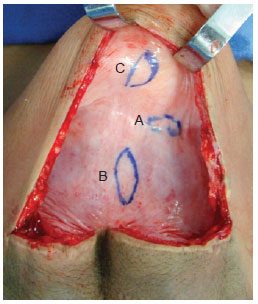
Figure 1 - Capsule showing sites for removal of tissue samples. edge (A), base (B), and dome (C).
Double-blinded histological analyses and thickness measurements of the histological specimens were all performed by a single observer, who was an anatomical pathologist in the UFRJ University Hospital Anatomical Pathology Department. The pathologist was blinded to the expander surface texture, the expansion time, the insertion plane, and whether the specimen was from a primary or re-expansion procedure.
Microscopic examinations, focusing on the inflammatory response, were performed on 114 specimens from 38 capsules to determine histological characterization of fragments from the base, edge, and dome of the expanded capsules. Samples from the expanded flap area, which we refer to as the capsule dome, were also analyzed with a 4× lens, marked in millimeters, to measure thickness (Figure 2). All segments were well oriented in the histological field, minimizing the effects of technical artifacts such as tissue folds. Measurements were taken moving from the innermost to the outermost part of the sample. Thickness results were expressed in millimeters, with each point on the lens corresponding to 0.09 mm.
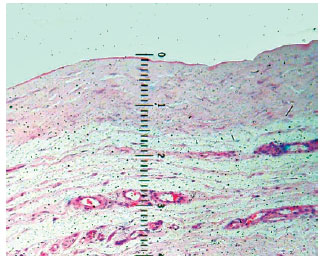
Figure 2 - Photomicrograph showing measurement details with millimeter lens. The loose connective tissue was not included in the measurement.
A Pearson's correlation test was used to evaluate the correlation between capsule thickness and expansion time. Analysis of variance (ANOVA), for samples with parametric distribution, was used to compare mean capsule thickness between the three insertion planes.
Unpaired Student's t tests were used to compare mean capsule thickness between smooth and textured expanders, subcutaneous and subfascial insertion planes, primary and re-expansion procedures, and flexible and rigid anatomic buttress (e.g., underlying muscle vs. bone). The difference in expansion time between flexible and rigid anatomic buttress was also compared using this test.
RESULTS
Results of Histopathological Samples Analyses from the Edge (A), Base (B), and Dome (C) of the Smooth and Textured Expander Capsules
Histopathological analyses focused on connective tissue characteristics of the expander capsule and on the associated inflammatory responses. The capsule wall largely consisted of dense, hyalinized connective tissue with highly eosinophilic collagen fibers. Loose connective tissue on the deep edge of the material, which contained nerve fibers and dilated vessels, was considered as accompanying tissue and not as part of the capsule wall itself. The connective tissue in the capsule wall showed relatively homogeneous density. In some cases, the innermost portion of the wall consisted of loose connective tissue, which was associated with the presence of macrophages on the capsule's inner edge.
The associated inflammatory response consisted mainly of macrophages, with lymphocytes and some eosinophils, observed in 62% of cases. Macrophages were arranged in a relatively ordered array on the capsule's internal edge and were referred to as "carpeting." Carpeting was observed in the majority of samples (71.5%) from textured expander capsules. In smooth expanders, carpeting was also observed, but in a more focal array. In cases where the macrophage "carpeting" appearance was not observed, the inner edge of the capsule had deposition of an amorphous, highly eosinophilic, delicately arranged material (fibrin, Figure 3). Macrophages also formed granulomata of the foreign body type, with the formation of multinucleated giant cells. These structures were present at various depths of the capsule wall.A foreign body-type granulomatous reaction was found in 54% of fragments and was occasionally associated with the presence of refringent material (foreign body) observed in the multinucleated giant cell cytoplasm (Figure 4).
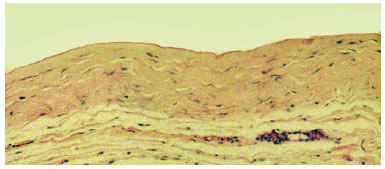
Figure 3 - Photomicrograph of a histological section showing the capsule wall, with dense connective tissue, mild inflammatory reaction, and collagen fibers displayed parallel to the inner surface. Note the delicate amorphous and eosinophilic material deposited on the inner edge (fibrin) (objective x10).
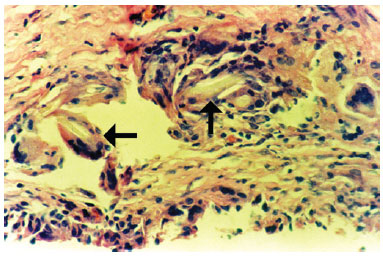
Figure 4 - Photomicrograph of a histological section showing the capsule wall, with numerous foreign body-type giant cells containing refringent material in their cytoplasm (foreign body) (objective x40).
No statistically significant differences were observed in histological characteristics between capsules formed around smooth expanders and those formed around rough ones.
Results Regarding to Capsule Thickness of the Expanders
There was no correlation between capsule thickness and expansion time (r = 0.262). Data from both smooth and textured expanders were pooled and a Pearson's correlation test was used to test statistical significance. There was also no statistical difference in capsule thickness between expanders placed subcutaneously and those placed subfascially (p = 0.16). Data from both types of expanders were again pooled and statistical significance was determined with a Student's t test.
The mean thickness between the 3 different insertion planes (subcutaneous, subfascia, and submuscular) showed a significant difference (ANOVA, p = 0.01). However, we attribute this difference to the fact that only 3 of the expanders studied were placed in the submuscular position.
Evaluation of capsule thickness for smooth and textured expanders using the Student's t test showed that the difference between the maximum and minimum thickness was statistically the same for the two types of surfaces (p = 0.48).
When the capsule thickness was compared between primary and re-expansion procedures, there was no statistical difference (p = 0.42). Moreover, there was no difference in capsule thickness (p = 0.88) when expanders were placed over rigid and flexible anatomic buttresses (e.g., bone vs. muscle). Data from smooth and textured expanders were pooled and the Student's t test was used to determine statistical significance.
DISCUSSION
The advent of tissue expanders has facilitated the repair of various congenital malformations and acquired lesions. Using expanders can result in less trauma, fewer sequelae, and improved functional and aesthetic outcomes. Tissue expanders can be used in a variety of locations throughout the body and in patients of all ages. Unfortunately, there has been limited publicity about expanders and using them can increase care cost and treatment time. Therefore, this procedure is used relatively infrequently, even though it often provides considerable benefit.
Manufacturers may launch a scientifically proven product that offers advantages over the products already in the market. These often come at higher cost, with price differences between smooth and textured expanders ranging from 41% to 56%14.
Studying capsule thickness in the expanded flap is important because the textured expanders produce thinner and more distensible capsules, with less pain, during the expansion process15-17. In a study in rabbits by Bern et al., capsules with textured expanders were more rigid4. In agreement with these findings, Wickman et al.18 observed thicker capsules with textured expanders than with smooth ones in post-breast reconstruction patients. However, there was wide variation in their capsule thickness measurements. Adams et al.1 found opposite results in rabbit studies that compared capsules formed around smooth and textured expanders.
We observed no significant difference in the mean thickness of capsules formed around subcutaneously and subfascially placed expanders. Interestingly, there was statistical difference when the expander was placed in the submuscular plane. We could find no clinical justification for this statistical difference, which may have been due to the small number of submuscular expanders. We also observed no significant histological differences between the three areas of placement or between the types of expander surfaces.
Several studies have detected different characteristics in capsules formed around smooth and textured implants. Some have described thick capsules with heavy cellularity around textured implants, while others have shown completely opposite results1. Results are also inconsistent between animal and human studies1.
The capsule generally forms with an inner layer next to the expander. This layer consists of dense connective tissue, fibroblasts, macrophages, histiocytes, eosinophils, and collagen fiber bands arranged parallel to the surface. An outer capsule layer also develops and is made up of loose connective tissue and abundant vascularization. Pasyk et al.19 studied 17 patients and found 4 histologically distinguishable zones in the capsule. Neither the body location nor capsule placement site was specified. Other authors have described 3 histological layers; the inner layer (in contact with the expander) with fibrinoid material and macrophages arranged in a palisade, a middle layer with dense connective tissue and parallel collagen fibers, and an outer layer with loose connective tissue and heavy vascularization2,7,20.
We also found only 3 distinct layers of the capsule. The inner layer had orderly arranged macrophages, described as a "carpeting" or palisade appearance. This was found, in 71.5% of all cases and more frequently in capsules from textured expanders. In capsules from smooth expanders, this appearance was more focal in many cases. When "carpeting" was not observed, the capsule's inner layer consisted of an amorphous, highly eosinophilic material such as fibrin. The intermediate layer mainly consisted of dense, hyalinized connective tissue with highly eosinophilic collagen fibers and, in some cases, consisted of loose connective tissue. In the outermost layer, highly vascularized, loose connective tissue was considered as accompanying tissue and not as part of the capsule wall itself.
Foreign body granulomata were seen in capsules from procedures with both smooth and textured expanders, but more frequently with smooth expanders. Some were found in the more superficial layer, while others were found in the deeper layers. These variations are in agreement with the findings of other studies15,17,19,20.
Also in agreement with our findings, Kostakoglu et al.20 observed refringent material in a foreign body granuloma. We frequently observed eosinophils in our samples, especially in capsules around smooth expanders. Such cells were observed in both primary and re-expansion procedures, without any apparent reason for their presence. Studies, in both animals and humans, have shown that eosinophils are present in capsules. Unfortunately, no explanations for or theories about their role or association with other factors were provided4,7,19,20. We believe that the presence of alloplastic material and the individual immune response to these materials may explain the presence of eosinophils, especially in patients who are hypersensitive to the expander material.
CONCLUSIONS
In conclusion, our findings showed no significant histological or thickness differences in capsules surrounding smooth and rough tissue expanders. We also found no clinical evidence that one type of expander is beneficial over another in the tissue expansion process.
REFERENCES
1. Adams WP Jr, Haydon MS, Raniere J Jr, Trott S, Marques M,1. Adams WP Jr, Haydon MS, Raniere J Jr, Trott S, Marques M, Feliciano M, et al. A rabbit model for capsular contracture: development and clinical implications. Plast Reconstr Surg. 2006;117(4):1214-21.
2. Horibe EK, Horibe K, Valente YS. Estudio experimental de la formación de la cápsula en piel expandida: contribución histológica. Cir Plast Iberolatinoam. 1989;15:267-75.
3. Caffee HH. Textured silicone and capsule contracture. Ann Plast Surg. 1990;24(3):197-9.
4. Bern S, Burd A, May JW. The biophysical and histologic properties of capsules formed by smooth and textured silicone implants in the rabbit. Plast Reconstr Surg. 1992;89(6):1037-44.
5. Heymans M, Lengele B, Lahlali N, Vanwijck R. A peri-implant capsule flap. Br J Plast Surg. 1993;46(6):456-9.
6. Bucky LP, Ehrlich HP, Sohoni S, May JW Jr. The capsule quality of saline-filled smooth silicone, textured silicone, and polyurethane implants in rabbits: a long-term study. Plast Reconstr Surg. 1994;93(6):1123-33.
7. Wieslander JB, Wieslander M. Prefabricated (expander) capsule-lined transposition and advancement flaps in reconstruction of lower eyelid and oral defects: an experimental study. Plast Reconstr Surg. 2000;105(4):1399-407.
8. Friedman HI, Friedman AC, Carson K. The fate of the fibrous capsule after saline implant removal. Ann Plast Surg. 2001;46(3):215-21.
9. Nahas FX, Vasconez LO, Ferreira LM. Guinea pigs as experimental model to evaluate the resistance of the tissue expander capsule. Acta Cir Bras. 2004;19(Suppl 1):96-103.
10. Gancedo M, Ruiz-Corro L, Salazar-Montes A, Rincón AR, Armendáriz-Borunda J. Pirfenidone prevents capsular contracture after mammary implantation. Aesthetic Plast Surg. 2008;32(1):32-40.
11. Tavares Filho JM. Expansores. In: Franco T, ed. Princípios de cirurgia plástica. São Paulo: Atheneu; 2002.p.275-87.
12. Tavares Filho JM, Claudio-da-Silva CS, Souza FZ. Uso de expansores de tecidos nos membros inferiores. Rev Col Bras Cir. 2005;32(6):290-6.
13. Tavares Filho JM, Belerique M, Franco D, Porchat CA, Franco T. Tissue expansion in burn sequelae repair. Burns. 2007;33(2):246-51.
14. Manders EK. Fire! Ready! Aim! Ann Plast Surg. 1994;32:232-3.
15. Barone FE, Perry L, Keller T, Maxwell P. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg. 1992;90(1):77-86.
16. May JW Jr, Bucky LP, Sohoni S, Ehrlich HP. Smooth versus textured expander implants: a double-blind study of capsule quality and discomfort in simultaneous bilateral breast reconstruction patients. Ann Plast Surg. 1994;32(3):225-33.
17. Copeland M, Choi M, Bleiweiss IJ. Silicone breakdown and capsular synovial metaplasia in textured-wall saline breast prostheses. Plast Reconstr Surg. 1994;94(5):628-36.
18. Wickman M, Johansson O, Olenius M, Forslind B. A comparison of the capsules around smooth and textured silicone prostheses used for breast reconstruction. A light and electron microscopic study. Scand J Plast Reconstr Surg Hand Surg. 1993;27(1):15-22.
19. Pasyk KA, Argenta LC, Austad ED. Histopathology of human expanded tissue. Clin Plast Surg. 1987;14(3):435-45.
20. Kostakoglu N, Keçik A, Özylmaz F, Safak T, Özgur F, Gursu G. Expansion of fascial flaps: histopathologic changes and clinical benefits. Plast Reconstr Surg. 1993;91(1):72-9.
1. Doctor and Master in Plastic Surgery at the Universidade Federal do Rio de Janeiro (Federal University of Rio de Janeiro) - UFRJ, full member of Sociedade
Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery) - SBCP, Rio de Janeiro, RJ, Brazil.
2. Doctor, Associate professor of Dermatology at UFRJ, Rio de Janeiro, RJ, Brazil.
3. Doctor, Adjunct Professor of Plastic Surgery at UFRJ, full member of SBCP, Rio de Janeiro, RJ, Brazil.
4. Resident in Plastic Surgery at UFRJ, aspiring member of SBCP, Rio de Janeiro, RJ, Brazil.
5. Doctor, Full Professor in Plastic Surgery at UFRJ, full member of SBCP, Rio de Janeiro, RJ, Brazil.
Correspondence to:
João Medeiros Tavares Filho
Rua Buenos Aires, 255 - Centro
Petrópolis, RJ, Brazil - CEP 22438-033
E-mail: jmedeiro@compuland.com.br
Article submitted to SGP (Sistema de Gestão de Publicações/ Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: February 11, 2012
Article accepted: May 8, 2012
Study conducted at the Hospital Universitário da Universidade Federal do Rio de Janeiro (University Hospital of the Federal University of Rio de Janeiro), Rio de Janeiro, RJ, Brazil.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license











