

Original Article - Year 2011 - Volume 26 -
Filling the nasal dorsum with Gore-tex in rhinoplasties
Uso do Gore-tex para preenchimento do dorso nasal em rinoplastias
ABSTRACT
Background: Many autogenous and exogenous materials have been frequently used for the production of grafts and implants in rhinoplasties. The ideal graft or implant should be biocompatible, biointegrated, non-absorbable, and easily moldable and should not cause an inflammatory response. Gore-tex, an expandable form of polytetrafluoroethylene (PTFE) has been used since the 1970s for vascular graft production. Although Gore-tex is extremely versatile and has extensive uses and low complication rates, the demonstration of Gore-tex use in aesthetic surgery is very limited in medical literature. Methods: We performed a retrospective study of 7 patients who received Gore-tex implants in order to fill the nasal dorsum from January 2005 to December 2007. All patients were assessed for aesthetic and functional factors and for the presence or absence of complications. Results: All patients had good postoperative evolution, with great satisfaction in terms of aesthetic and functional aspects and no complications. Conclusions: Gore-tex is a satisfactory synthetic material as it is inexpensive, easily moldable, has good biocompatibility, and has shown no incidence of extrusion or infection in implants used for nasal dorsum filling in previously reported cases.
Keywords: Rhinoplasty. Polytetrafluoroethylene. Nose/surgery.
RESUMO
Introdução: Muitos materiais autógenos e exógenos vêm sendo utilizados com frequência para confecção de enxertos e implantes nas rinoplastias. O enxerto ou implante ideal deve ser biocompatível, biointegrável, não-absorvível, facilmente moldável e não deve causar resposta inflamatória. O Gore-tex, uma forma expansível do politetrafluoretileno (PTFE), é usado desde a década de 1970 na confecção de próteses vasculares. Apesar de seu uso ser extremamente versátil e extenso, oferecendo baixas taxas de complicação, a demonstração na literatura médica de seus resultados, quando usado na cirurgia estética, é muito restrita. Métodos: Realizado estudo retrospectivo sobre a evolução de 7 pacientes submetidos a implantes de Gore-tex visando ao preenchimento do dorso nasal, no período de janeiro de 2005 a dezembro de 2007. Todos os pacientes foram avaliados quanto a fatores estéticos e funcionais, e quanto à presença ou não de complicações. Resultados: Todos os pacientes tiveram boa evolução pós-operatória, com grande satisfação do ponto de vista estético e funcional, e sem complicações. Conclusões: O Gore-tex mostrou-se um material sintético satisfatório, de baixo custo, facilmente moldável, com boa biocompatibilidade e com incidência nula de extrusão ou infecção para implantes de preenchimento de dorso nasal nos casos apresentados.
Palavras-chave: Rinoplastia. Politetrafluoretileno. Nariz/cirurgia.
Many autogenous and exogenous materials have been used for the production of grafts and implants in rhinoplasties.
The ideal graft or implant should be biocompatible, biointegrated, non-absorbable, and easily moldable and should not cause an inflammatory response.
Autogenous cartilage, especially septal cartilage, is still the most appropriate graft material for use in rhinoplasty, as it has biocompatibility, long-term stability and a low complication rate. The major limitation is its poor availability, as in cases of major trauma, secondary rhinoplasty, and non- Caucasian noses with relatively low and slightly convex nasal dorsa. In these cases, an alloplastic material is required for the implant1,2.
Several alloplastic materials were used in the past in nasal surgery, such as Silastic, Proplast, and Plastipore. These materials had high rates of migration, reabsorption, extrusion, and infection. Other materials, such as Mersilene and Supramid, were difficult to remove when necessary3.
Polytetrafluoroethylene (PTFE) is widely accepted in the field of facial plastic surgery and reconstructive surgery4. The material has micropores with sizes ranging from 10 µm to 30 µm, into which host cells penetrate, promoting suitable fixing of the implant and enabling easy removal when necessary. Results have showed that this material is, biocompatible, biointegrated, and does not induce inflammatory reactions in animal models1.
Gore-tex, an expandable form of PTFE, was developed by Gore in mid-1960 and has been used since the 1970s for the manufacture of vascular grafts5,6. This material was approved for use in facial plastic surgery in the United States by the Food and Drug Administration (FDA) in 1993 7.
Although this material is extremely versatile and extensive, offering low complication rates, demonstration of its use is very limited in medical literature.
Here, we report our experience with Gore-tex for nasal dorsum filling.
METHODS
This is a retrospective study on the postoperative evolution of 7 patients who received Gore-tex implants to fill the nasal dorsum at Hospital Universitário São José and at Clínica Notre Dame (Belo Horizonte, MG) from January 2005 to December 2007.
All patients were assessed for subjective aesthetic and functional factors and for the presence or absence of complications.
The procedures were performed with the patient under general anesthesia (5 patients) or intravenous sedation (2 patients), with infiltration of an anesthetic solution with adrenaline in the area to be operated to enhance local hemostasis.
Exo-rhinoplasty was performed for 5 patients; endo-rhinoplasty for 1 patient; and for the remaining patient, an incision was made over a traumatic scar on the nasal dorsum.
Rhinoplasty was primary in 5 cases and secondary in 2 cases. Osteotomy was performed for 4 patients.
Each Gore-tex implant was prepared manually; the material was cut into progressively less wide sheets in order to form a pyramid and was stabilized with absorbable sutures (Figure 1).

Figure 1 - Polytetrafluoroethylene prepared for implant.
Perioperative antibiotic prophylaxis was performed with cephalothin.
The implant was introduced through the periosteal and subperichondral tunnels with minimal dissection; this ensured implant stability without fixing with sutures.
Adhesive tapes (1 month) and plaster splint (1 week) were used postoperatively for 7 and 4 patients, respectively.
Patients were followed-up for 2 to 5 years, and the outcome measures included patient satisfaction and the desired aesthetic and functional benefits. We also conducted medical assessments of the aesthetic improvement, technical considerations, complications, and the preoperative and postoperative photographic documentation.
RESULTS
The patients' ages ranged from 19 to 46 years (average, 28.2 years). All patients showed good evolution in the postoperative period and were satisfied in terms of aesthetics and functionality (Figures 2 to 5).
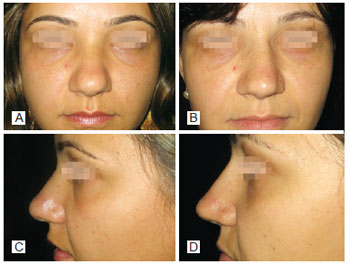
Figure 2 - In A, preoperative period of primary rhinoplasty with the use of polytetrafluoroethylene (PTFE) in the nasal dorsum, front view. In B, postoperative period of primary rhinoplasty with the use of PTFE in the nasal dorsum, front view. In C, preoperative period of primary rhinoplasty with the use of PTFE in the nasal dorsum, left lateral view. In D, postoperative period of primary rhinoplasty with the use of PTFE in the nasal dorsum, left lateral view.
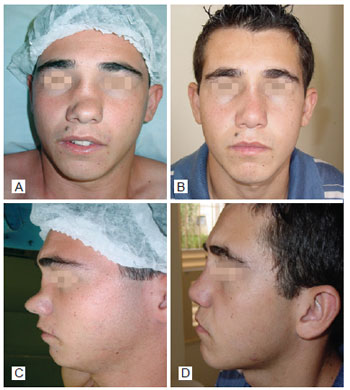
Figure 3 - In A, preoperative period of post trauma rhinoplasty with the use of polytetrafluoroethylene (PTFE) in the nasal dorsum, front view. In B, postoperative period of primary rhinoplasty with the use of PTFE in the nasal dorsum, front view. In C, preoperative period of primary rhinoplasty with the use of PTFE in the nasal dorsum, left lateral view. In D, postoperative period of post trauma rhinoplasty with the use of PTFE in the nasal dorsum, left lateral view.
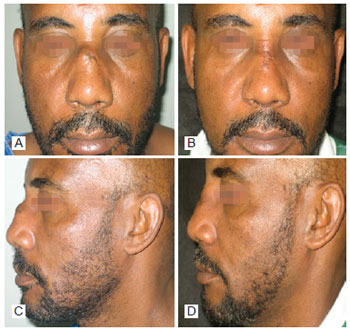
Figure 4 - In A, preoperative period of post trauma rhinoplasty with the use of polytetrafluoroethylene (PTFE) in the nasal dorsum, front view. In B, postoperative period of post trauma rhinoplasty with the use of PTFE in the nasal dorsum, front view. In C, preoperative period of post trauma rhinoplasty with the use of PTFE in the nasal dorsum, left lateral view. In D, postoperative period of post trauma rhinoplasty with the use of PTFE in the nasal dorsum, left lateral view.
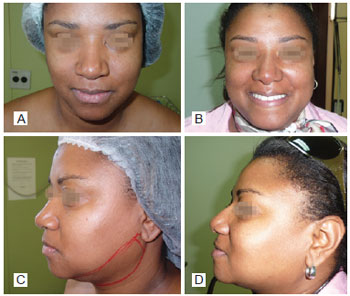
Figure 5 - In A, preoperative period of primary rhinoplasty with the use of polytetrafluoroethylene (PTFE) in the nasal dorsum, front view. In B, postoperative period of primary rhinoplasty with the use of PTFE in the nasal dorsum, front view. In C, preoperative period of primary rhinoplasty with the use of PTFE in the nasal dorsum, left lateral view. In D, postoperative period of primary rhinoplasty with the use of PTFE in the nasal dorsum, left lateral view.
There were no complications such as infection, reaction to foreign body, and graft extrusion or migration in any case in this study. The implant was perceptible by palpation in only 2 patients. No patient had a visually perceptible implant.
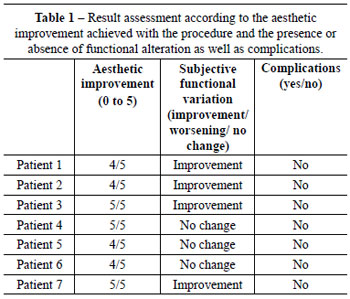
For assessment of results, a table was created with scores from 0 to 5, according to the aesthetic improvement achieved with the procedure and the presence or absence of functional alterations and complications (Table 1). The grades correspond to the aesthetic assessment made by the surgeon, the presence or absence of early and late surgical complications, and subjective functional assessment reported by patients. The primary objective of the procedure is aesthetic improvement with no change in or improvement of function.
DISCUSSION
Gore-tex use is widespread in other surgical specialties such as vascular surgery, in which millions of grafts have been employed, with more than 20 years of monitoring. No case of rejection or carcinogenesis has been reported. Gore-tex has also been widely used in hernia and abdominal wall surgeries and vaginal and rectal prolapses.
The combination of minimal inflammatory response with a tendency to gradually increase its stability over time are 2 excellent features of Gore-tex implants, as demonstrated in the study of Maas et al.8 in an animal model.
Owsley and Taylor5 noted no complications among 106 patients who used Gore-tex implants for a period exceeding 5 years. In 1999, Godin et al.4 monitored 309 patients for an average of 40.4 months and reported a complication rate of 3.2%. Another study, published by Conrad and Gillman9, reported 7 instances of complications in 189 patients followed-up for 17.5 months.
Schoenrock and Reppucci10 demonstrated the importance of avoiding direct contact between the graft and dermis, as this approach might predispose to increased inflammatory reaction and graft rejection.
Complications such as infections or foreign body reactions may occur with Gore-tex use. However, there was no evidence of resorption or changes in shape and contour in cartilage and bone grafts. In cases where an infection is non-responsive to antibiotic therapy, the Gore-Tex implant should be removed. According to Godin et al.4 and Conrad and Gillman9, implant removal can be easily performed without resulting in permanent deformity.
Grafts or implants, especially synthetic ones, should be used with caution in secondary rhinoplasty, as scarring and changes in lymphatic drainage may predispose the patient to complications.
Patient selection in this study was based on the need for nasal dorsum filling, regardless of whether the rhinoplasty was primary or not. The choice of Gore-tex for the implant was based on safety, the low rate of complication in published literature, and ease of use. The features of Gore-tex, such as ease of use and a low learning curve for surgery, justify its use, since it is a safe implant, provides excellent nasal contour, and requires minimal surgical time.
CONCLUSION
Gore-tex is a satisfactory synthetic material, as it is inexpensive, easily moldable, has good biocompatibility, and has shown no incidence of extrusion or infection in implants used for nasal dorsum filling in reported cases. The surgeon must weigh factors such as skin density, technical difficulty, and degree of deformity before suggesting and implementing the use of Gore-tex, as is true when considering any other implant.
Long-term studies are needed to determine complication rates and success in a larger number of patients for establishing precise criteria and indications for the use of Gore-Tex in nasal surgery.
REFERENCES
1. Inanli S, Sari M, Baylancicek S. The use of expanded polytetrafluoroethylene (Gore-Tex) in rhinoplasty. Aesthetic Plast Surg. 2007;31(4):345-8.
2. Ortiz Monasterio F, Michelena J. The use of augmentation rhinoplasty techniques for the correction of the non-Caucasian nose. Clin Plast Surg. 1988;15(1):57-72.
3. Conrad K, Torgerson CS, Gillman GS. Applications of Gore-tex implants in rhinoplasty reexamined after 17 years. Arch Facial Plast Surg. 2008;10(4):224-31.
4. Godin MS, Waldman SR, Johnson CM Jr. Nasal augmentation using Gore-Tex: a 10-year experience. Arch Facial Plast Surg. 1999;1(2):118-21.
5. Owsley TG, Taylor CO. The use of Gore-tex for nasal augmentation: a retrospective analysis of 106 patients. Plast Reconstr Surg. 1994;94(2):241-8.
6. Soyer T, Lempinen M, Cooper P, Norton L, Eiseman B. A new venous prosthesis. Surgery. 1972;72(6):864-72.
7. W. L. Gore & Associates. Gore-tex soft tissue patch: technical bulletin. Flagstaff: W. L. Gore & Associates; 1994.
8. Maas CS, Gnepp DR, Bumpous J. Expanded polytetrafluoroethylene (Gore-tex soft tissue patch) in facial augmentation. Arch Otolaryngol Head Neck Surg. 1993;119(9):1008-14.
9. Conrad K, Gillman G. A 6-year experience with the use of expanded polytetrafluoroethylene in rhinoplasty. Plast Reconstr Surg. 1998;101(6):1975-83.
10. Schoenrock LD, Reppucci AD. Correction of subcutaneous facial defects using Gore-Tex. Facial Plast Surg Clin North Am. 1994;2:373-87.
1. Plastic surgeon, assistant professor of plastic surgery at Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.
2. Plastic surgeon, member of the Brazilian Society of Plastic Surgery (SBPC), Belo Horizonte, MG, Brazil.
3. Resident practitioner of the Department of Plastic Surgery, Hospital Universitário São José (HUSJ), Belo Horizonte, MG, Brazil.
Correspondence to:
Rodrigo Otávio Gontijo Tostes
Rua Primavera, 112/302 - Santo Antônio
Belo Horizonte, MG, Brazil - CEP 30330-260
E-mail: rtostes@terra.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Received: June 15, 2011
Accepted: August 13, 2011
Study conducted at Hospital Universitário São José and at Clínica Notre Dame, Belo Horizonte, MG, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter