

Original Article - Year 2011 - Volume 26 -
Siliconomas
Siliconomas
ABSTRACT
Introduction: The use of industrial liquid silicone for correcting defects and for soft-tissue augmentation is associated with several complications such as infection, tissue necrosis, and product migration through the lymphatic and/or venous systems or even by gravity. Objective: The main objective of the present study was to clarify the harmful effects that this product may have on the body, such as major scars and aesthetic and functional sequelae. Methods: Eleven patients with siliconomas (7 men and 4 women, including 3 transgender individuals) were treated at the Hospital Universitário, Universidade Federal de Juiz de Fora or at the Clínica Plastic Center, Minas Gerais, Brazil, between January 2005 and December 2010. Conservative treatment was attempted through systemic administration of corticosteroids and steroidal infiltration into the nodules. The surgical treatments ranged from direct resection of nodules to elevation of skin flaps and skin grafting. Patients with extensively affected body area underwent liposuction, and a patient with breast involvement underwent bilateral subcutaneous mastectomy followed by silicone gel implantation as a single surgical procedure. Results: All the patients reported that the material had been injected into their bodies by lay individuals in nonhospital settings. The most common affected organs were the eyelids, malar and deltoid regions, breasts, buttocks, and lower limbs. Each patient underwent 1-3 surgical procedures, depending on the extent and location of the affected area. Histopathological examination of the resected material revealed no malignancy, and the material removed by liposuction was confirmed to be silicone. Conclusions: Monitoring of early and late complications shows that the treatment is long, and multiple surgical procedures are necessary to correct functional and aesthetic sequelae.
Keywords: Silicone oils/adverse effects. Silicone oils/toxicity. Silicones.
RESUMO
Introdução: O silicone líquido industrial tem sido introduzido no organismo humano de forma clandestina, com a finalidade de corrigir defeitos, depressões, irregularidades e para aumentar volumes, tanto em mulheres como em homens ou transgêneros. Com tal uso, podem ocorrer várias complicações, tais como infecções, necroses teciduais e, mais tardiamente, a migração do produto, pelo sistema linfático, venoso ou mesmo pela força da gravidade. Método: Foram avaliados 11 pacientes portadores de siliconomas, pertencentes ao ambulatório do Serviço de Cirurgia Plástica do Hospital Universitário da UFJF e da Clínica Plastic Center, em Juiz de Fora, MG, no período de janeiro de 2005 a dezembro de 2010. Desses, 7 eram do gênero masculino e quatro do gênero feminino, sendo que desse total, 3 eram transgêneros. Todos os pacientes referiam com clareza o material injetado em seus organismos e todos fizeram o procedimento em ambiente não hospitalar e por indivíduos leigos. Conclusão: Este artigo tem como objetivo maior esclarecer as consequências danosas que esse produto pode causar no organismo, como cicatrizes de grandes proporções e sequelas estéticas e funcionais.
Palavras-chave: Óleos de silicone/efeitos adversos. Óleos de silicone/toxicidade. Silicones.
Industrial liquid silicone has been clandestinely introduced in the human body for correcting defects and for soft-tissue augmentation. Despite its harmful effects1,2, it is applied on a large scale in places with poor hygiene, in the absence of asepsis and antisepsis.
The early complications of liquid silicone injection, such as infections and tissue necrosis, are usually derived from application without basic asepsis and antisepsis, and the late effects occur because of product migration to other body regions through the lymphatic and/or venous systems or even by gravity. Injectable liquid silicone induces a typical foreign body reaction, which may be encapsulated, that subsequently forms a calcified fibrosis, acquiring a hard and stony consistency with irregularities, and sometimes presents as skin dyschromia when superficially located.
The main objective of the present study is to characterize the early and late complications of liquid silicone, which include large scar formation and aesthetic and functional sequelae. We also propose a strategy for managing these adverse effects when silicone is injected in the breasts, eyelids, and in the malar, gluteal and deltoid regions in men, women, and transgender individuals. The use of this material is no longer restricted to homosexual and transgender individuals but is also being used by heterosexual men and women.
History
Since antiquity, humans have been interested in improving their looks and body contour, in an attempt to adapt to prevailing beauty standards. Over the years, several innovations were presented and misused as alternatives to achieve the desirable attributes. However, these alternatives are not always safe or free from complications. For example, in the early twentieth century, Gersuny2 tried to use paraffin injections for breast enlargement; this technique fell into disuse because of major complications.
Over the past 30 years, silicone has played a key role in improving aesthetics. Silicone comprises an inorganic skeletal structure of silicium atoms bound to oxygen atoms or chains and lateral organic groups of hydrocarbons3. The 3 basic forms of silicone are liquid, gel, and elastomer. The apparent simplicity and low cost of liquid silicone has increased its indiscriminate use.
The term "silicone" refers to the polymers containing manufactured elemental silicone. These polymers are a family of compounds with a wide variety of physical and chemical properties, purity, sterility, and biocompatibility. Injectable liquid silicone or polydimethylsiloxane fluid, which is the product used to fill tissue, consists of repeated units of dimethylsiloxane with a terminal trimethylsiloxane4.
Liquid silicone was introduced in the early twentieth century, for aesthetic and reconstructive purposes, and was very popular in Japan in the 1950s; soon thereafter came the first reports of adverse reactions and complications, especially with the illicit use of injections of this substance by unqualified persons2,5. Between 1960 and 1970, physicians and lay people worldwide used silicone oil or industrial silicone injections for breast augmentation and body contouring. Within a few years (3-20 years), most of the treated individuals developed serious complications ranging from product migration to carcinoma6. The technique was therefore abandoned and its use was prohibited by the American Food and Drug Administration (FDA) and the Brazilian DIMEP2.
METHODS
Between January 2005 and December 2010, 11 patients with siliconoma (4 women and 7 men, including 3 transgender individuals) were treated at the Hospital Universitário, Universidade Federal de Juiz de Fora or at the Clínica Plastic Center, Minas Gerais. The patients ranged in age from 25 years to 68 years. The most common affected organs were the eyelids, malar and deltoid regions, breasts, buttocks, and lower limbs. All the patients reported that the material was injected into their body by lay individuals in nonhospital settings. The most prevalent clinical manifestations were allergies induced by immune reactions, foreign body granulomas, and deformities caused by displacement of liquid silicone. The diagnosis was essentially clinical. Some patients underwent additional tests such as radiography, ultrasonography, magnetic resonance imaging, laboratory tests, and routine cardiac evaluation.
In some cases, conservative treatment was attempted through systemic administration of corticosteroids and infiltration of steroids into the nodules. This management is consistent with previous reports of granulomatous reactions showing good responses to a variety of therapies, including the use of very potent topical steroids and antibiotics7,8. The predominant management was application of systemic corticosteroids (10-20 mg/day prednisone) during periods of allergic exacerbation. In cases of severe granulomatous reactions, triamcinolone was injected into the most visible nodules. No patients were treated in the acute phase of infection or skin necrosis.
All the patients were finally treated by surgery ranging from direct resection of nodules to skin flap removal and skin grafting. Some patients with extensively affected body areas underwent liposuction to partially remove the injected liquid silicone9. A patient with breast involvement underwent bilateral subcutaneous mastectomy followed by silicone gel implantation as a single surgical procedure.
Figures 1-7 illustrate some of the cases.
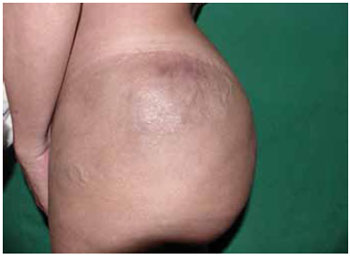
Figure 1 - Siliconoma in the left trochanteric region.
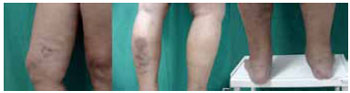
Figure 2 - Siliconoma in the lower limb. Liquid silicone applied to the thigh that has migrated to the calf and malleolus.
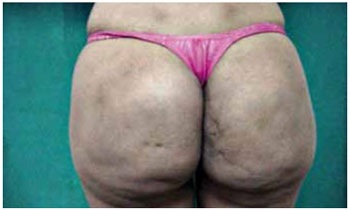
Figure 3 - Siliconoma in the gluteal region.
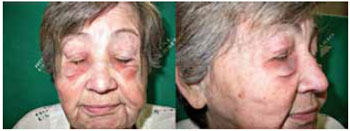
Figure 4 - Siliconoma in the eyelids.
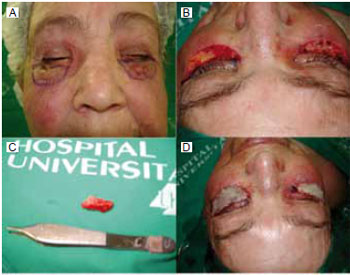
Figure 5 - A: Marking for surgical resection of siliconoma in the lower eyelid. B: Completely resected area. C: Surgical specimen of siliconoma from the lower eyelid. D: Total skin graft in the lower eyelid.
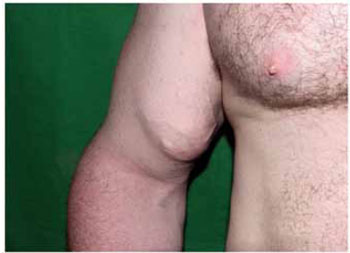
Figure 6 - Siliconoma in the right biceps.
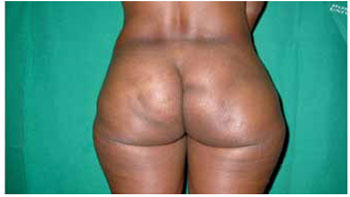
Figure 7 - Siliconoma in the gluteal region.
RESULTS
The number of surgical procedures per patient ranged from 1 to 3, depending on the extent and location of the affected area.
All the resected materials were examined histopathologically and no malignancy was observed in the affected areas. The material removed by liposuction was also sent for pathological examination, confirming it to be silicone (Figures 8-10).
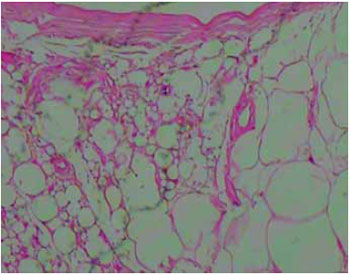
Figure 8 - Image of the subcutaneous tissue showing round cystic spaces containing nonbirefringent translucent material (HE stain, X100 magnification).
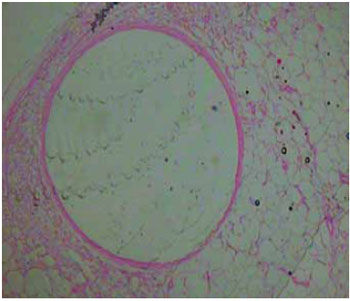
Figure 9 - Image of the subcutaneous tissue displaying a large cystic space containing nonbirefringent translucent material (HE stain, X100 magnification).
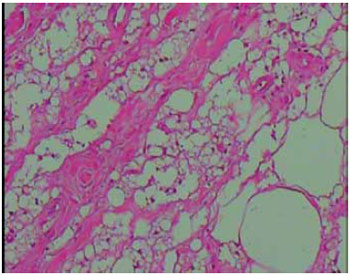
Figure 10 - Image of the subcutaneous tissue showing round cystic spaces containing nonbirefringent translucent material (HE stain, X100 magnification).
DISCUSSION
The term "siliconoma" was popularized in 1965 to characterize the foreign body reaction similar to that described for other liquids such as paraffin10. It is the most common finding of mastopathy due to silicone, and is probably related to the expansion and growth of breast cancer because it has been observed as an abnormal opening of the lymphatic channels close to the sites of granulomas and silicone migration, including lymph nodes6,11,12.
Silicone has an irregular surface and eventually forms granulomas. Microspheres and particles smaller than 15 μm are generally phagocytosed and can be transported to lymph nodes. Larger microspheres of nonabsorbable polymers, with smooth surfaces, become encapsulated in fibrous tissue and escape phagocytosis. Clinically, all fluids and injected particles cause foreign body reactions to varying degrees. Until the mechanism of granuloma formation is fully understood, the possibility of its late development is not predictable4,13.
The adverse effects and complications of injectable liquid silicone as a filling material can be acute such as edema, ecchymosis, erythema, and dyschromia, or chronic such as overcorrection, embolism, granulomatous reactions, idiosyncratic reactions, migration, and connective tissue disease7,8. Several authors have described complications with the use of injectable silicone in the breasts13-15. Ficarra et al.16 reported complications in several patients who received liquid silicone injections in the face. Further, Garcia Diez et al.17 reported the case of a patient who injected silicone into his penis, and Astudillo et al.18 reported a case of infiltration of liquid silicone in the buttocks. None of the patients in the present series had siliconomas in the genital area.
At present, many individuals, both men and women, use alloplastic substances to change the aesthetic appearance of their genitalia. As liquid silicone is easily obtainable at low cost, we believe that its usage will increase.
Monteiro et al.15 reported the case of a male patient who requested the removal of injected silicone in his breasts to remove stigma and recover his masculine body contour.
Vargas et al.19 studied 10 patients, including 9 women, who used liquid silicone injections. They reported that this procedure is more often used by transgender individuals to make their bodies more feminine. However, according to the present series, the profile of these patients seems to be changing, as the procedure was also widely used by heterosexual men and women.
The most common clinical presentations in the present cases were immune responses such as allergies, which recurred; foreign body granulomas, which were painful or not but caused more or less intense nodular deformities, according to their location; and deformities caused by displacement.
Conservative treatment was the first choice, with systemic administration of corticosteroids in cases of allergy and local infiltration of steroids in granulomas7,8.
Allergic reaction was the most prevalent manifestation, followed by facial contour deformity, decreased facial movement, and difficulty closing the eyelids. In cases of deformity due to silicone migration or infection, surgery for partial removal of the substance and tissue remodeling were indicated. Some cases of siliconoma in the face can be treated with CO2 laser, which seems to reduce the scarring sequelae20.
Ideally, a substance for soft-tissue contouring should be safe, biocompatible, stable after implantation, nonmigratory, resistant to phagocytosis, persistent, maintain its volume without being resorbed or degraded, induce minimal foreign body reaction, nonteratogenic, noncarcinogenic, noninfectious, nontoxic, nonallergenic, not require testing before use, autologous, not cause pain or discomfort, have low cost, and be suitable for storage at room temperature. Liquid silicone is far from meeting these requirements and is therefore not suitable therapeutically.
The demand to restore volume loss that occurs with the aging process has stimulated the emergence of numerous filling substances, and a larger number of more suitable substances are available today. Historically, the use of silicone has been proven to be dangerous, and, at present, sequelae of past treatments with this substance can still be seen4.
CONCLUSION
Despite the small sample size, the profile of the patients in the present study is different from that reported in the literature. Although the most recent cases are of heterosexual men and women, a vast majority of the cases used to involve transgender and homosexual individuals.
The present report shows that the treatment of the early and late complications of liquid silicone use is long and associated with multiple surgical procedures, with functional and aesthetic sequelae, which somehow seems to discourage its use. Although these individuals desire immediate and long-lasting aesthetic results at a low cost, the use of permanent implants must be performed under the supervision of well-trained specialists.
REFERENCES
1. Andrews JM, Haddad CM, Ramos RR, Martins DMFS, Ferreira LM. Morbidade e mortalidade após injeção de silicone líquido em seres humanos. Folha Méd. 1989;99(2):69-72.
2. Freitas RJ, Cammarosano MA, Rossi RHP, Bozola AR. Injeção ilícita de silicone líquido: revisão de literatura a propósito de dois casos de necrose de mamas. Rev Soc Bras Cir Plast. 2008;23(1):53-7.
3. Ortiz-Monasterio F, Trigos I. Management of patients with complications from injections of foreign materials into the breasts. Plast Reconstr Surg. 1972;50(1):42-7.
4. Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9(2): 133-40.
5. Hage JJ, Kanhai RC, Oen AL, van Diest PJ, Karim RB. The devastating outcome of massive subcutaneous injection of highly viscous fluids in male-to-female transsexuals. Plast Reconstr Surg. 2001; 107(3):734-41.
6. Frisina AA, Pedrosa CF, Gomes KS. Pacientes com siliconomas e sua associação com tumores nas mamas. Rev Soc Bras Cir Plást. 2005; 20(2):117-9.
7. Orentreich DS, Jones DH. Silicone líquido injetável. In: Carruthers J, Carruthers A, eds. Técnicas de preenchimentos. Série procedimentos em Dermatologia Cosmética. Rio de Janeiro:Elsevier;2005. p.79-94.
8. Duffy D. Complications of fillers: overview. Dermatol Surg. 2005;31(11 Pt 2):1626-33.
9. Morgenstern L, Gleischman SH, Michel SL, Rosenberg JE, Knight I, Goodman D. Relation of free silicone to human breast carcinoma. Arch Surg. 1985;120(5):573-7.
10. Broder KM, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 Suppl):7S-14S.
11. Athre RS. Facial filler agents. Op Tech Otolaryngol. 2007;18:243-7.
12. Hans-Georg E. An introduction to polymer science. 2nd ed. New York: VCH;1997.
13. Brown SL, Silverman BG, Berg WA. Rupture of silicone-gel implants: causes, sequelae and diagnosis. Lancet. 1997;350(9090):1531-7.
14. Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006;118(3 Suppl):77S-84S.
15. Monteiro E, Stuque CO, Freitas RJ, Bozola AR. Exérese de silicone injetável em mamas masculinas: abordagem cirúrgica. Rev Bras Cir Plást. 2010;25(2):404-7.
16. Ficarra G, Mosqueda-Taylor A, Carlos R. Silicone granuloma of the facial tissues: a report of seven cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(1):65-73.
17. García Díez F, Izquierdo García FM, Benéitez Álvarez ME. Granuloma de silicona en el pene "siliconoma". Arch Esp Urol. 2005;58(5): 457-60.
18. Astudillo CA, Díaz JJ, Silva CFA, Catalán V, Agurto PU. Caso radiológico. Rev Chilena Radiol. 2005;11(3):150-3.
19. Vargas AF, Amorim NG, Pitanguy I. Complicações tardias dos preenchimentos permanentes. Rev Bras Cir Plást. 2009;24(1):71-81.
20. Chui CH, Fong PH. Carbon dioxide laser vaporization of facial siliconomas: flash in the pan or way of the future? Ann Plast Surg. 2008;60(3):272-5.
1. Plastic Surgeon, Full Member of the Brazilian Society of Plastic Surgery (BSPS), Juiz de Fora, MG, Brazil.
2. Plastic Surgeon, Full Member of the BSPS, Head of Plastic Surgery, University Hospital, Federal University of Juiz de Fora, Juiz de Fora, MG, Brazil.
3. Medical Student, Federal University of Juiz de Fora (UFJF), Juiz de Fora, MG, Brazil.
4. Plastic Surgeon, Specialist Member of the BSPS, Juiz de Fora, MG, Brazil.
5. Resident Physician at the Department of Plastic Surgery from UFJF, Juiz de Fora, MG, Brazil.
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Received: January 9, 2011
Accepted: February 25, 2011
Work performed at the Hospital Universitário da Universidade Federal de Juiz de Fora (HU-UFJF) and at the Clínica Plastic Center (University Hospital, Federal University of Juiz de Fora (HU-UFJF) and at the Clinical Plastic Center), Juiz de Fora, MG, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter