

Case Report - Year 2025 - Volume 40Issue 1
Breast Implant-Associated Anaplastic Large Cell Lymphoma: A Case Report
Linfoma anaplásico de células grandes associado a implante mamário: Um relato de caso
ABSTRACT
Although breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a disorder still sparsely described in the literature, the number of reported cases is rising rapidly. Few of the cases published to date involve Brazilian patients.We present a case treated at Instituto Nacional de Câncer (INCA) with an emphasis on the plastic surgery follow-up, which included serial fat-grafting procedures to restore breast volume without new implants. The prognosis of BIA-ALCL is often favorable, provided its diagnosis and treatment are accomplished early. The present report aims to contribute to the recognition and management of this condition in plastic surgery practices.
Keywords: allografts; plastic surgery; anaplastic large-cell lymphoma; breast implants; breast
RESUMO
O linfoma anaplásico de células grandes associado a implante mamário (BIA-ALCL, do inglês breast implant-associated anaplastic large cell lymphoma) é um distúrbio ainda pouco descrito na literatura; porém, o número de casos está crescendo rapidamente. Dos casos que foram publicados até agora, poucos são de pacientes brasileiras. Apresentamos um caso tratado no Instituto Nacional de Câncer (INCA) com ênfase no seguimento realizado pela cirurgia plástica, que incluiu lipoenxertias seriadas para restauração do volume mamário sem uso de novos implantes. O prognóstico do BIAALCL associado a implantes mamários é geralmente favorável, desde que diagnosticado e tratado precocemente. Este relato visa contribuir para o reconhecimento e abordagem dessa doença na prática da cirurgia plástica.
Palavras-chave: aloenxertos; cirurgia plástica; implante mamário; linfoma anaplásico de células grandes; mama
Introduction
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a T-cell lymphoma. The first case report dates from 1997.1 As of June 30, 2024, a total of 1,380 cases have been described worldwide.2 The epidemiology of BIA-ALCL is limited by the lack of data on the prevalence of implant users, type of implants, and associated adverse events, challenging estimates of incidence and risk. To date, virtually all cases described are related to textured implants, with a mean time to onset of 8 to 9 years after implantation. Diagnosis relies on clinical features, including delayed effusion, mass, swelling, pain, rash, or cutaneous ulcer. Diagnostic confirmation requires cytology and seroma immunophenotyping and/or immunohistochemistry (IHC).3
Objective
The present article aims to report a clinical case of BIA-ALCL, highlighting the diagnostic process, surgical treatment (explantation and capsulectomy), and oncological followup. In addition, we demonstrate the reconstructive strategy adopted (breast fat grafting) and emphasize the significance of early recognition of this condition for a better prognosis.
Materials and Methods
The present descriptive observational study is a case report from Instituto Nacional de Câncer (INCA), Rio de Janeiro, RJ, Brazil. Information collection used the following:
Review of the patient’s medical records, including clinical and surgical data and supplementary exams (imaging and histopathology).
Direct observation of clinical evolution in the preand postoperative periods.
Bibliographic review in databases such as Public Medical Database (PubMed), Scientific Electronic Library Online (SciELO), and Google Scholar, using descriptors in Portuguese and English (BIA-ALCL, breast implants, and anaplastic large cell lymphoma).
Informed Consent Form for image use and clinical information dissemination with no patient identification.
The institution’s Ethics Committee approved this study under opinion number 7.515.140.
We did not use any sampling method as this is a single case described in depth to illustrate the clinical presentation, treatment, and oncological follow-up.
Results
Case Report
A 45-year-old HIV-positive patient was undergoing antiretroviral therapy with dolutegravir, darunavir, and ritonavir, and had no other comorbidity. She had history of breast augmentation in 2014 and reported a sudden, painless increase in the size of the right breast starting in August 2021. A fine-needle aspirationbiopsy ofseroma in the right breast revealed the presence of markedly atypical cells of lymphoid lineage and was positive for malignancy.
After discussing the case with the mastology team in March 2022, we decided on bilateral explantation with capsulectomy for histopathological diagnosis.
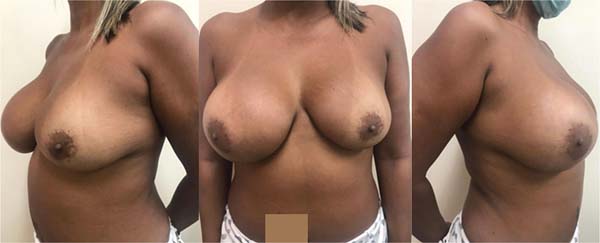
In March 2022, the physical examination revealed a right breast slightly larger than the left, breast implants without contracture, absence of palpable nodules, and a fibroelastic lymph node in the right armpit (►Fig. 1).
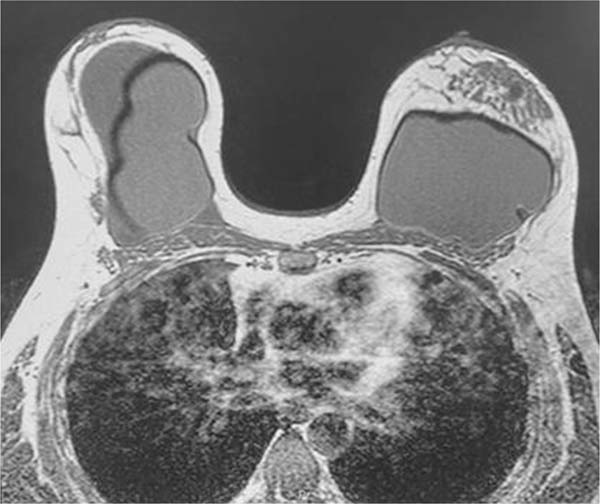
Magnetic resonance imaging showed an extensive intracapsular collection in the right breast with heterogeneous content, associated with enhancement and thickening of the fibrous envelope and capsule, consistent with an inflammatory process and capsular contracture. It was not possible to rule out BIA-ALCL. The Breast Imaging-Reporting and Data System (BIRADS) was 4 (►Fig. 2).
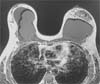
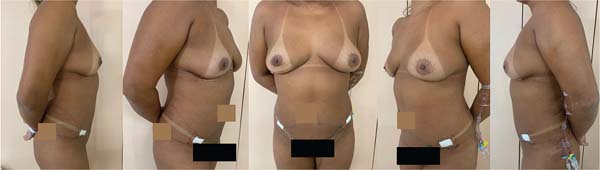
On May 2, 2022, a surgical procedure removed the implants (textured device) and involved total capsulectomy. Evolution in the immediate postoperative period was adequate (►Fig. 3).
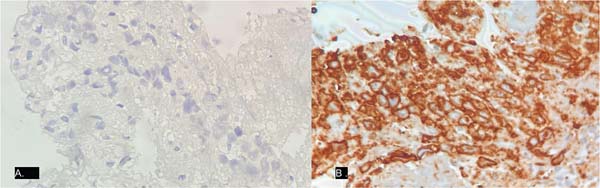
The histopathological study report of the specimen (►Figs. 4-5) read as follows: “Anaplastic large cell lymphoma associated with breast implant. The cells line the capsule. Absence of capsular infiltration or solid mass formation. Associated giant-histiocytic foreign body reaction. Immunohistochemistry was positive for CD30, CD4, CD3, CD2, and granzyme B and negative for ALK, CD20, and CD8.”
The patient had a satisfactory postoperative evolution and remains under follow-up by the INCA oncology and hematology teams. Her monitoring includes blood and imaging tests (positron emission tomography-computed tomography [PET-CT]), showing no evidence of active disease or need for adjuvant therapy to date.
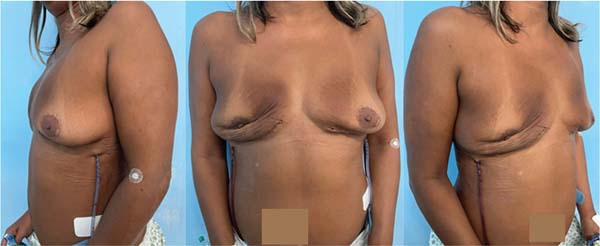
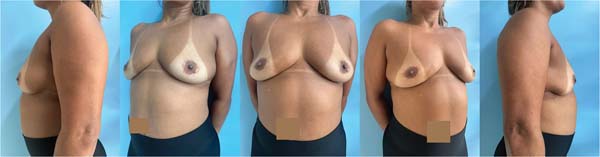
After postoperative recovery, the INCA plastic surgery team recommended multiple serial fat-grafting sessions for the patient to increase breast volume. The patient did not wish to undergo a mastopexy surgery or exhibit extensive scars.
The first fat-grafting session occurred in September 2023, and the second session occurred in early December 2024 (►Figs. 6-7).
The patient reports satisfaction with the breast volume obtained after fat grafting. She remains under follow-up with oncology, mastology, hematology, and plastic surgery clinics and presents no evidence of new lesions.
Discussion
According to estimates, more than two million Brazilian women have breast implants, making Brazil the world’s second-largest market for these devices. Nevertheless, there are few reports of BIA-ALCL in Brazilian patients.4
The etiology of BIA-ALCL is not clear. However, according to the literature, the most common processes for BIA-ALCL development include bacterial contamination of rough/microtextured surface implants or surface-related friction. These processes would culminate in a persistent immune response, subsequently progressing to lymphoma. Several examples support the theory that cancer may result from infection by organisms such as Helicobacter pylori and the Epstein-Barr virus.5 Furthermore, there are no reports of BIA-ALCL cases before the textured implant era, even when silicone fluids migrated to surrounding tissues and produced granulomas.6
In most cases, BIA-ALCL manifests as late seroma, which occurs 7 to 10 years after implant surgery. Therefore, the onset of a seroma a year or more after implant surgery that cannot be explained by trauma should be considered suspicious for the disease.
Approximately 10 to 40% of patients diagnosed with BIAALCL may develop a mass, cutaneous manifestations, capsular contracture, and lymphadenopathy.7 In our patient, the sudden increase in size of the right breast was the only evident change.
Diagnosis relies on imaging tests and cytological, IHC, and immunophenotypic analysis of seroma, revealing the presence of large, pleomorphic cells with eosinophilic cytoplasm. The IHC pattern includes CD30 protein expression and absence of anaplastic lymphoma kinase (ALK) translocation protein expression.8
Ultrasound is recommended as the first test, followed by aspiration of seroma fluid for immunocytochemistry and cytopathological analysis. The protocol may include computed tomography and magnetic resonance imaging if the ultrasound findings are unclear.
Rare cases, presenting a mass rather than fluid, require a pathological analysis of fragments originating from the capsular mass and skin. In some cases, a pathological evaluation of suspected lymph nodes may detect potential metastases. Our case had no signs of extramammary disease.
Treatment consists primarily of surgical removal of the breast implants and capsule. The National Comprehensive Cancer Network (NCCN) protocol recommends that plastic surgeons confirm the diagnosis of ALCL before surgery.4 Another critical point is the removal of both implants and capsules, as there are cases of disease onset in the other breast, and replacement with textured implants is not recommended.9,10 Typical margins used in traditional breast cancer surgery are not necessary. After complete excision of the capsule and implants, surveillance exams should occur every 3 to 6 months, in addition to periodic imaging tests, including chest, abdominal, and pelvic CT or PET-CT every 6 months for the first 2 years. If complete excision is not technically feasible or if surgical pathology reveals incomplete excision or partial capsulectomy, local radiotherapy is often used asadjuvant therapy in the absence of systemic disease.11In more advanced cases, the patient should be monitored by an oncologist who may perform a lymphadenectomy. Chemotherapy and radiation therapy may also be treatment alternatives.
Breast implant-associated anaplastic large cell lymphoma has a good prognosis, and the complete removal of the capsule is a determining factor for cure. It is also crucial to have an appropriate preoperative plan and treatment in place to prevent the progression of this lymphoma. Reports of death in BIA-ALCL patients are associated with late diagnosis or more aggressive forms of the disease, including tumor nodules, lymph node involvement, bilateral breast involvement, and capsule infiltration.
Breast reconstruction after BIA-ALCL treatment is feasible and can be considered in properly selected patients. The decision regarding the timing and method of reconstruction depends on the extent of the disease at the time of diagnosis, the completeness of surgical excision, and the patient’s preferences and risk factors. Reconstruction options include smooth implant placement, mastopexy, autologous flaps, and fat grafting.10
Our patient underwent implant removal and bilateral capsulectomy. Both units were textured round implants in a subglandular position. After surgery, the patient was monitored by INCA’s hematology and oncology teams, with no indication for adjuvant therapy to date, and is satisfied with the plastic surgery team’s treatment plan.
Conclusion
We conclude that BIA-ALCL is a relatively new but highly relevant disease. It requires increasing awareness among patients and the medical community to increase knowledge of its diagnosis and treatment.
References
1. Groth AK, Clemens MW, Graf R, Sebastião AP, Azambuja AP, Louveira MH, et al. Breast Implant-Associated Anaplastic Large-Cell Lymphoma in Brazil: What Are We Missing? Plast Reconstr Surg 2022;150(05):970e-978e. Doi: 10.1097/PRS. 0000000000009652
2. United States Food and Drug Administration (FDA). Medical Device Reports of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Silver Spring, MA: FDA; 2024 [cited 2025 Mar 22]. Available from: https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma
3. Batista BN, Garicochea B, Aguilar VLN, Carvalho FM, Millan LS, Fraga MFP et al. Relato de caso de linfoma anaplásico de células grandes associado a implante mamário em paciente brasileira. Rev Bras Cir Plástica 2017;32(Suppl 3):445-449 Doi: 10.5935/ 2177-1235.2017RBCP0073
4. Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant- Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J 2019;39(Suppl 1):S3-S13. Doi: 10.1093/asj/sjy331
5. Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 1997;100(02): 554-555. Doi: 10.1097/00006534-199708000-00065
6. Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, Andersen JS, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 2015;135(03):695-705. Doi: 10.1097/PRS. 0000000000001033
7. Groth AK, Graf R. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and the Textured Breast Implant Crisis. Aesthetic Plast Surg 2020;44(01):1-12. Doi: 10.1007/s00266-019-01521-3
8. Klassen AF, Cano SJ, Grotting JC, Baker SB, Carruthers J, Carruthers A, et al. FACE-Q Eye Module for Measuring Patient-Reported Outcomes Following Cosmetic Eye Treatments. JAMA Facial Plast Surg 2017;19(01):7-14. Doi: 10.1001/jamafacial.2016.1018
9. National Comprehensive Cancer Network (NCCN). NCCN Guidelines: T-Cell Lymphomas. Plymouth Meeting, PA: NCNN; 2024 Available from: https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf
10. Lamaris GA, Butler CE, Deva AK, Miranda RN, Hunt KK Connell T, et al. Breast Reconstruction Following Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):51S-58S. Doi: 10.1097/ PRS.0000000000005569/
11. Leberfinger AN, Behar BJ, Williams NC, Rakszawski KL, Potochny JD, Mackay DR, Ravnic DJ. Breast implant-associated anaplastic large cell lymphoma: A systematic review. JAMA Surg 2017;152 (12):1161-1168. Doi: 10.1001/jamasurg.2017.4026
1. Plastic Surgery Division, Instituto Nacional de Câncer, Rio de Janeiro, RJ, Brazil
2. Pathology Division, Instituto Nacional de Câncer, Rio de Janeiro, RJ, Brazil
Address for correspondence Dunia Verona, Divisão de Cirurgia Plástica, Instituto Nacional de Câncer, Rio de Janeiro, RJ, Brazil (e-mail: veronadunia@gmail.com duniaverona@hotmail.com).
Artigo submetido: 28/03/2025.
Artigo aceito: 14/07/2025.
Conflict of Interests
The authors have no conflict of interests to declare.









 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter