INTRODUCTION
Microsurgical procedures are characterized by the application of a set of
techniques and surgical maneuvers on millimetric anatomical structures carried
out with the aid of optical magnifying lenses
1
. Its principles can be applied to various medical specialties, such as in
the areas of reconstructive plastic surgery, oncology, urology, head and neck,
and transplants
2
. General surgeons can also apply its principles to biliodigestive or bile
duct anastomoses or to uncomplicated vascular reconstructions
3
. It is a complex technique that requires great manual skill and
continuous surgical training before application in humans
2
.
In the literature, there are several training models described involving
different materials, both of animal and non-animal origin. Each model has
particularities, advantages, and disadvantages. In the training of initial basic
techniques, most descriptions use non-animal models, such as latex
4 , 5
. When improving microscopic surgical skills, animal models are preferred,
such as mice, pigs, birds and dogs, depending on the availability of each institution
6 - 9
.
Practices using synthetic materials that mimic organic tissue for the development
of basic skills, followed by the use of models with tissues of animal origin,
rationalize animal use and reduce costs in the initial stages of learning
10
. The experimental models developed for basic training in microsutures
must enable the teaching of experimental surgery safely, allowing the handling
of instruments for microsurgery and the surgical microscope
4
.
In many countries, there are no training centers or facilities that have adequate
technical resources for learning in this area. In this context, the biggest
obstacle to achieving adequate training becomes the necessary investment.
Therefore, the development of alternative experimental models for the practice
of microsurgical techniques is essential. With the diversity of models, each
center can choose or adapt the one that best fits their routine and their
possibilities in relation to costs
11
.
OBJECTIVE
The objective of this work is to propose a training model for microsurgical
sutures based on the use of remaining, discarded segments of bovine pericardium
patch, a material used in vascular repairs in humans.
METHOD
This work was submitted and analyzed by the Animal Use Ethics Committee of the
Agricultural Sciences Sector of the Universidade Federal do Paraná
(CEUA-SCA-UFPR) and deemed exempt from approval due to proof of the origin of
the biological material sold, manipulated by the biomedical industry, certified
by the National Health Surveillance Agency (ANVISA).
The creation of the microsurgical suture training model based on a fragment of
bovine pericardium patch ( Figure 1 ) uses
a surgical microscope, microclamp, scissors, straight and curved tweezers,
microsurgical needle holder, as well as threads and materials of synthesis. The
material used in this model is the remains of the open bovine pericardium plate,
although not completely used, in carrying out vessel repairs in vascular
surgeries in humans, which would be discarded.
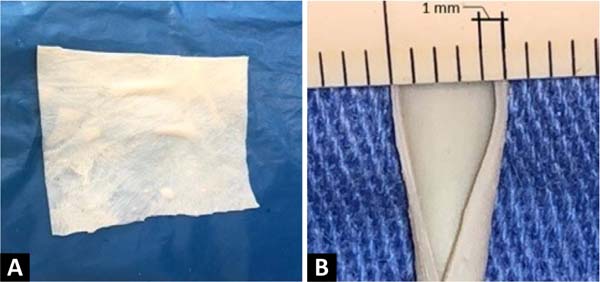
Figure 1 -
A: Bovine pericardium patch plaque. B:
Thickness of the bovine pericardium patch (0.5mm) and its
layers.
Figure 1 -
A: Bovine pericardium patch plaque. B:
Thickness of the bovine pericardium patch (0.5mm) and its
layers.
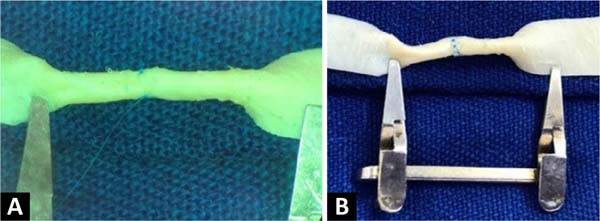
The procedure begins by cutting out a small segment of the bovine pericardium
measuring 60mm x 6.8mm, fixing one of its edges to the surgical field using a
clamp, and positioning the whole under the lenses of the surgical microscope.
An
incision is made in the middle of the plate, dividing it into two identical
segments ( Figure 2 ). Two tubes are then
made at each end, with the help of separate simple stitches, with
Prolene®8-0 applied to join the upper and lower edges of each
segment.
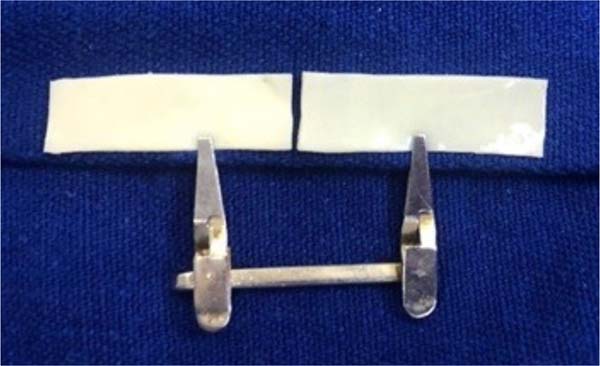
Figure 2 - Fragment of bovine pericardium measuring 30mm x 6.8mm each, after
divided in half, forming two independent plates fixed to the
surgical field using a microsurgical clamp.
Figure 2 - Fragment of bovine pericardium measuring 30mm x 6.8mm each, after
divided in half, forming two independent plates fixed to the
surgical field using a microsurgical clamp.
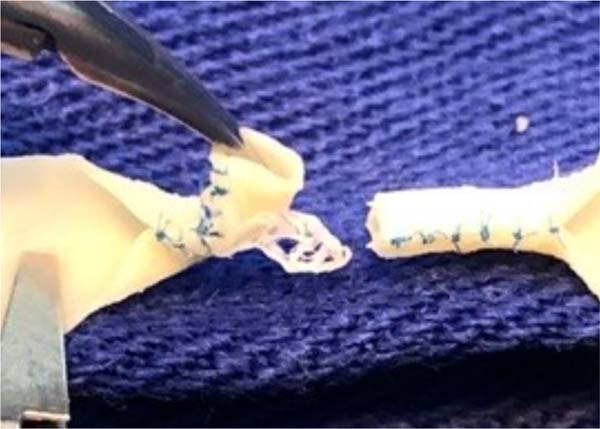
At the end of this step, two free circular ends are obtained, which mimic the
sectioned ends of a 2.0 mm diameter vessel ( Figure 3 ). The outer edges of both segments of this section were
prepared in the same way that vascular structures are prepared for microsuture,
removing the excess adventitial layer to prevent the entry of excess tissue into
the future vascular lumen during training in creating the anastomosis ( Figure 4 ).
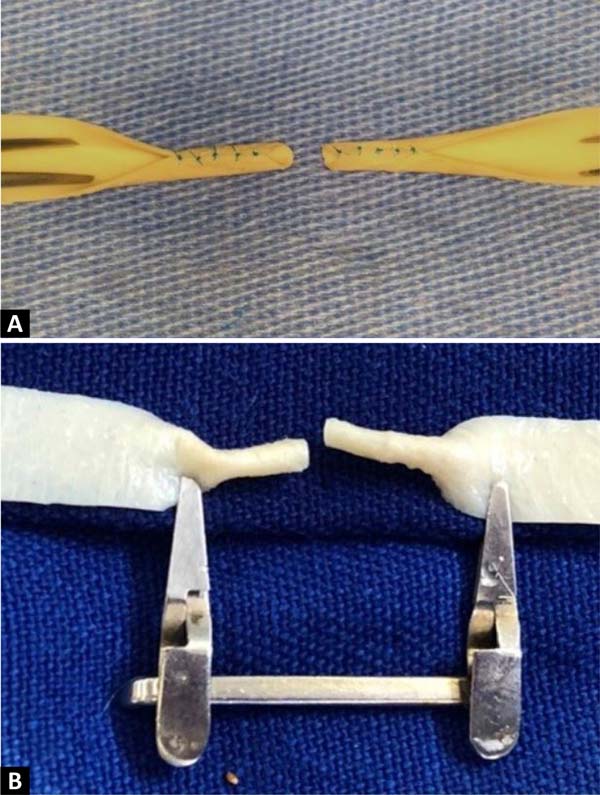
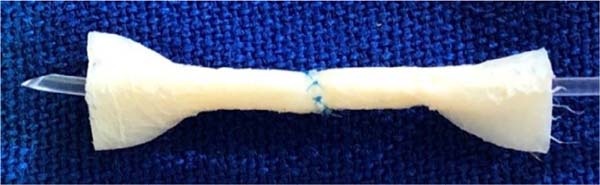
Figure 3 -
A: Upper margins of each fragment sutured to the lower
margins, forming two 2.0mm diameter tubes, mimicking terminal
vascular stumps, to create an end-to-end anastomosis. B: The same
structure in the posterior view, fixed to the surgical field with
clamps.
Figure 3 -
A: Upper margins of each fragment sutured to the lower
margins, forming two 2.0mm diameter tubes, mimicking terminal
vascular stumps, to create an end-to-end anastomosis. B: The same
structure in the posterior view, fixed to the surgical field with
clamps.
Figure 4 - Excess of the adventitial layer being removed to avoid the
accidental entry of redundant tissue into the lumen of the vascular
model during the end-to-end anastomosis.
Figure 4 - Excess of the adventitial layer being removed to avoid the
accidental entry of redundant tissue into the lumen of the vascular
model during the end-to-end anastomosis.
At this point, the model is ready to create an end-to-end microvascular
anastomosis of the two ends that mimics the lumen of a small vessel. The
vascular anastomosis can be created with the aid of an 8-0
Prolene®thread, creating two anchoring points opposite at 180° and
followed by simple stitches separated between the two initial anchoring points
(
Figure 5 ).
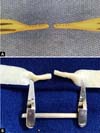
Figure 5 -
A: Two initial anchoring points of the end-to-end
anastomosis, opposite at 180º. B: Microsurgical
end-to-end anastomosis completed.
Figure 5 -
A: Two initial anchoring points of the end-to-end
anastomosis, opposite at 180º. B: Microsurgical
end-to-end anastomosis completed.
After suturing the anastomosis, it is possible to test the patency of the vessel
by introducing a number 20F vascular catheter inside it. Transposition without
resistance demonstrates the absence of accidental suturing of the posterior wall
( Figure 6 ).
Figure 6 - Passage of a catheter, number 20F, through the interior of the
vessel model with end-to-end anastomosis, demonstrating its
patency.
Figure 6 - Passage of a catheter, number 20F, through the interior of the
vessel model with end-to-end anastomosis, demonstrating its
patency.
RESULTS
With the alternative experimental model described, it was possible to train
microsuture and microanastomose techniques using bovine pericardium patch
plates. The model is reproducible in an experimental laboratory that has
apparatus and instruments for microsurgery. This is an economically viable
model, as it used leftover biological material widely used in surgeries and
vascular repairs, which would be discarded after the original surgical
procedure. In addition to the economic advantage, due to the use of fragments
of
biological material, the model did not involve the use of experimental
animals.
It was observed that bovine pericardium tissue presents the same drawbacks
present in vascular sutures in humans, such as the risk of layer delamination,
excess adventitial layer and the risk of suturing the posterior wall. With
direct visualization of all layers of the sutured tissue, dissection of the
excess adventitial layer, and passage of a catheter to test its patency, it was
possible to overcome these inconveniences.
DISCUSSION
Due to the great complexity, initial training in microsurgical procedures must
first be carried out in an environment that does not involve patients. The
microsurgical skills training routine must be defined in order first to become
familiar with the instruments, properly handle the surgical threads, and master
dissection, suturing and tissue anastomosis techniques. The practice of these
skills under adequate supervision, with a well-established routine and with the
use of materials that would otherwise be discarded, makes it possible to obtain
the bases for the initial training of academics, residents, and surgeons, as
it
allows the immediate application of theoretical knowledge in an experimental
model. and rationalizes animal use, reducing costs
6 , 12
.
Several experimental models for the practice of microsurgical techniques have
already been described in various animals, such as pigs, dogs, chickens, and
rats
6 , 8 , 9
. However, the strict institutional laws imposed by ethics committees on
animal use can hinder the broad use of animals in the learning process.
Artificial materials such as gloves or silicone tubes are also found as
alternative models in the literature
4 , 13 , 14
. However, these models may have the disadvantage of not perfectly
mimicking the vascular tissue, reducing the level of difficulty in learning
microanastomoses techniques
12
.
Training these techniques can be long and exhausting. In this way, by scaling the
technical difficulty, starting with synthetic materials, evolving to the
pericardial patch model, and, later, animal models, training can become
stimulating since the practical evolution of the training itself is perceived
8
.
The experimental model proposed here makes it possible to create anastomotic ends
mimicking vessels of different calibers, depending on the size of the plate that
is initially cut. In the described procedure, 8-0 Prolene thread was used for
a
structure with a diameter of 2.0 mm, obtained from a cut plate with a free end
of 6.8 mm. It is recommended to use a 9-0 thread if the diameter of the
anastomotic ends is less than 2mm.
One of the advantages of using surplus bovine pericardium patch material is
precisely its ability to mimic the concentric layers of vascular tissue. When
applying microsutures to this material, there is a risk of delamination or
detachment of the intimal layer when the suture partially transfixes the wall
thickness of the material. Care must be taken to cover the entire thickness of
the bovine pericardium wall when applying sutures during end-to-end anastomosis
training, and care must also be taken to keep the posterior wall free and not
suture it together with the points of the previous anastomosis line so that the
anastomosis is kept patent ( Figure 6
).
Before performing a microvascular anastomosis, the adventitia and periadventitial
tissues are routinely removed from the small arteries. This is one of the most
important parts of vascular anastomoses, as it allows a clear definition of
where the vessel ends and a more precise creation of the suture. Furthermore,
excess tissue from the adventitial layer, if close to the cut ends of the
vessel, can become interposed in the anastomosis and, as it is a highly
thrombogenic tissue, can lead to anastomosis failure. Non-animal microsurgical
models are unlikely to be able to mimic this important step
15
. This also provides training in removing the adventitial layer, a
standard procedure performed before a microvascular anastomosis ( Figure 3 ).
CONCLUSION
The use of bovine pericardium plates as a training model for sutures and
microsurgical end-to-end anastomoses allows the development of microsurgical
skills in a realistic way since the manipulation of this material mimics the
layers of a blood vessel and presents technical difficulties similar to those
human tissues.
The proposed model uses good quality material, which would otherwise be
discarded, which makes the implementation cost low when compared to models that
use simulators or animals. Furthermore, it avoids the inconveniences of animal
use, such as maintenance of breeding and storage facilities, food costs,
analgesia, and disposal, among others. Therefore, the use of surplus bovine
pericardium that would otherwise be discarded is an appropriate model of
microsurgical training in the initial phase of learning, as it has properties
very similar to human tissues and does not require animal use.
REFERENCES
1. Pessoa SGP, Riquet GF. Fundamentos básicos de microcirurgia
vascular: estudo experimental. Ceará Med.1982;4(1):10-6.
2. Martins PNA, Montero EF. Basic microsurgery training. Comments and
proposal. Acta Cir Bras. 2007;22(1):79-81.
3. Di Cataldo A, Li Destri G, Trombatore G, Papillo B, Racalbuto A,
Puleo S. Usefulness of microsurgery in the training of the general surgeon.
Microsurgery. 1998;18(8):446-8.
4. Dias IS, Pessoa SGP, Benevides AN, Macêdo JE. Treinamento inicial em
microcirurgia. Rev Bras Cir Plást. 2010;25(4):595-9.
5. Pessoa BBGP, Pessoa SGP. Treinamento em microanastomoses utilizando
tubos de látex. Acta Cir Bras. 2002;17(2):143-6.
6. Marcondes CA, Pessoa SGP, Pessoa BBGP, Dias IS, Guimarães MGM.
Padronização técnica no treinamento em microcirurgia do serviço de cirurgia
plástica e microcirurgia reconstrutiva do hospital universitário Walter Cantídio
da Universidade Federal do Ceará (HUWC/UFC). Rev Bras Cir Plást.
2010;29(2):283-8.
7. Kinshoku MR, Rodriguez CAL, Fidalgo RDS, Duran CCG, Leme PLS, Duarte
IDS. Uso racional de modelos animais para pesquisa e ensino de microcirurgia.
Rev Col Bras Cir. 2012;39(5):414-7.
8. Maluf Junior I, Silva ABD, Groth AK, Lopes MAC, Kurogi AS, Freitas
RS, et al. Modelo experimental alternativo para treinamento em microcirurgia.
Rev Col Bras Cir. 2014;41(1):72-4.
9. Pessoa BBGP, Pessoa SGP. O retalho hipogástrico cutâneo no cão:
modelo para o aprendizado experimental de microcirurgia. Acta Cir Bras.
2002;17(3):198-202.
10. Grober ED, Hamstra SJ, Wanzel KR, Reznick RK, Matsumoto ED, Sidhu
RS, et al. The educational impact of bench model fidelity on the acquisition
of
technical skill: the use of clinically relevant outcome measures. Ann Surg.
2004;240(2):374-81.
11. Isolan GR, Santis-Isolan PMB, Dobrowolski S, Cioato MG, Meyer FS,
Antunes ACM, et al. Considerações técnicas no treinamento de anastomoses
microvasculares em laboratório de microcirurgia. J Bras Neurocir.
2010;21(1):8-17.
12. Webster R, Ely PB. Treinamento em microcirurgia vascular: é
economicamente viável? Acta Cir Bras. 2002;17(3):194-7.
13. Hoşnuter M, Tosun Z, Savaci N. A nonanimal model for microsurgical
training with adventitial stripping. Plast Reconstr Surg.
2000;106(4):958-9.
14. Lima DA, Galvão MSL, Cardoso MM, Leal PRDA. Rotina de treinamento
laboratorial em microcirurgia do Instituto Nacional do Câncer. Rev Bras Cir
Plást. 2012;27(1):141-9.
15. Lohman R, Siemionow M, Lister G. Advantages of sharp adventitial
dissection for microvascular anastomoses. Ann Plast Surg.
1998;40(6):577-85.
1. Universidade Federal de São Paulo, São Paulo,
SP, Brazil
2. Universidade Federal do Paraná, Curitiba, PR,
Brazil
Corresponding author: Caroline Cunico Rua Botucatu, 740, 2º andar,
Vila Clementino, São Paulo, SP, Brazil, Zip Code: 04023-062, E-mail:
cunico.caroline@gmail.com
Article received: March 16, 2023.
Article accepted: June 13, 2023.
Conflicts of interest: none.