INTRODUCTION
Since its introduction two decades ago1,2, negative
pressure wound therapy (NPWT) has been established for its effectiveness in
managing acute and chronic wounds3-7. However, the
high technology makes the device complex to handle and expensive, reducing its
use in institutions with limited resources8. Trying to solve these problems, simplified vacuum
dressings systems (SVD)8-12 have been proposed since NPWT
does not necessarily require a special apparatus and can prepare wounds for
surgical treatment8,12-14. Despite using more basic mechanical and electrical
components, SVD retains essential safety attributes such as controlled suction
and wound sealing1,8,10,15,16.
Operational characteristics of SVD have been poorly evaluated and, occasionally,
seriously criticized3,16. Most of the studies available
do not have comparison groups and use limited methodologies, thus deserving
further evaluation8,10,11,15,17,18. The primary deficiencies are the use of rudimentary
materials, difficulty sealing wounds, and inability to maintain subatmospheric
pressures8,15,16,19. Deficiencies
result in accumulations of exudates, dressing changes, and repeated manipulation
of injuries. In addition to being boring, manipulations increase the risk of
aggravating injuries. In minor exudative wounds, inadequate seals can cause
perilesional air circulation, resulting in dryness, hemorrhage, and progressive
tissue necrosis1,19-21.
The decision to use a specific dressing should be guided not only by potential
efficacy, adverse effects, location, and symptoms of lesions (pain, exudate,
etc.) but also by the frequency of changes, clinical experience, patient
preference, and costs22-24. Even when economic value is
not an issue, the best treatment may be challenging to implement or unavailable,
so it is essential to know efficient second indication alternatives22.
OBJECTIVE
The study’s objective was to evaluate the feasibility (operational and financial)
of an SVD model (SVDM).
MATERIAL AND METHODS
Feasibility study based on a randomized superiority clinical trial, blinded, with
two parallel arms, carried out between January 1, 2017, and May 1, 2020, Roberto
Santos Hospital (RSH; teaching hospital, multidisciplinary, 640 beds - Salvador,
Bahia - Brazil). The trial was registered in the Brazilian Registry of Clinical
Trials (RBR-5c8y6v) and followed CONSORT 2010 recommendations25. The research was approved by
the Research Ethics Council of the RSH (CAAE 55556816.7.0000.5028) and performed
following the Declaration of Helsinki. An Informed Consent Form was obtained
from the participating patients.
A sample of 50 patients was calculated using the R statistical software (R Core
Team, 2018), assuming a mean expected success rate of 98% for the SVDM group
and
72% for the control group, with a margin of superiority of 25%. A test power
of
80% and a significance level of 5% were assumed. Patients were admitted
sequentially in treatment (SVDM) and control groups (hydrofiber silver - SHF,
Aquacel Ag+ Extra™ - Convatec Inc., ER Squibb & Sons, North Carolina - USA)
following a list of random numbers performed in the statistical software R. The
statistical analysis used was by treatment protocol.
Adult patients hospitalized for acute (< 3 months) or chronic (≥ 3 months)
wounds were included in the study. Subjects with decompensated systemic
disorders (cardiac, thyroid, renal, pulmonary, hepatic, arterial hypertension,
severe anemia, severe malnutrition, and coagulopathies) were excluded. Painful
wounds, infected wounds, injuries associated with perilesional dermatoses,
allergic reactions, malignant neoplasms, and exposure to underlying exposed
vessels, nerves, or viscera were also not included. The emergence of serious
complications (e.g., hemorrhage, allergic reactions, sepsis, extensive necrosis,
severe pain), decompensation of previously controlled systemic disorders, and
deaths not attributable to the dressings were exclusion criteria used.
Wounded areas were obtained using the SketchandCalc app
(www.sketchandcalc.com - Figure 1). Application, SVDM, and clinical examples are shown in Figures 2 to 4. SVDM was regulated with a pressure of -125 mmHg. The first
dressing was used in continuous mode and the others in intermittent mode (5
minutes of vacuum and 2 minutes without vacuum)2,26.
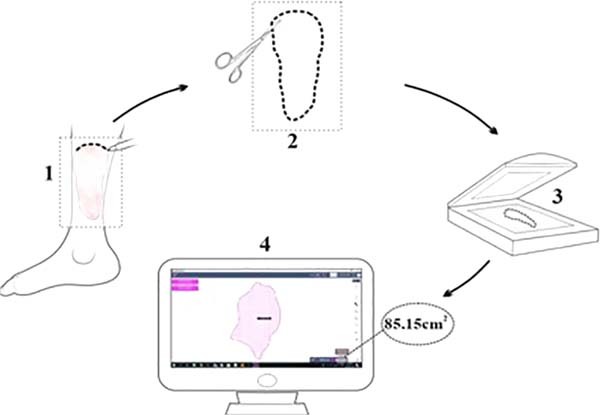
Figure 1 - Measurement of wounded areas. 1: design of lesion contours using
transparent acetate film, 2: clipping of the demarcated area,
obtaining a two-dimensional pattern (template), 3: digitalization,
4: computerized measurement of the injured area.
Figure 1 - Measurement of wounded areas. 1: design of lesion contours using
transparent acetate film, 2: clipping of the demarcated area,
obtaining a two-dimensional pattern (template), 3: digitalization,
4: computerized measurement of the injured area.
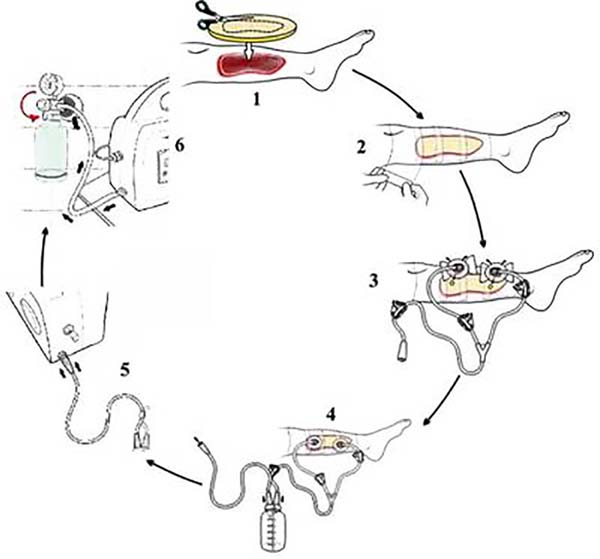
Figure 2 - Applying the SVDM. 1: cutting and placing the foam on the lesion,
2: sealing the foam using a transparent polyurethane adhesive film,
3: placing suction cups on one or two holes (2 cm) made in the film
on the foam, 4: suction tube connection to the liquid collection
canister, 5: connection of the canister to the control unit, 6:
activation of the NPWT and adjustment of the subatmospheric
pressure.
Figure 2 - Applying the SVDM. 1: cutting and placing the foam on the lesion,
2: sealing the foam using a transparent polyurethane adhesive film,
3: placing suction cups on one or two holes (2 cm) made in the film
on the foam, 4: suction tube connection to the liquid collection
canister, 5: connection of the canister to the control unit, 6:
activation of the NPWT and adjustment of the subatmospheric
pressure.
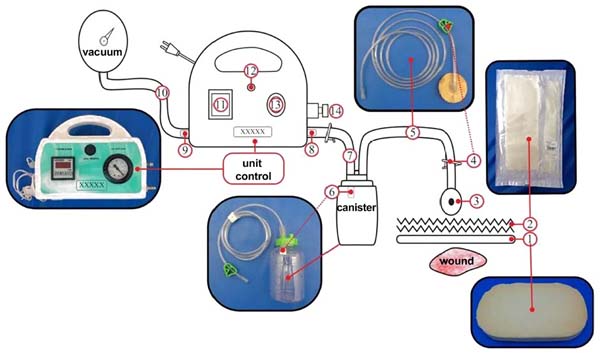
Figure 3 - SVDM setup. 1: foam, 2: adhesive film (polyurethane), 3: suction
cup, 4: clip cuts flow, 5: drainage tube, 6: filter, 7 and 10:
connecting tubes, 8: inlet and 9: air outlet, 11: timer display
(digital), 12: start button, 13: vacuum gauge display (pneumatic),
14: vacuum adjustment knob.
Figure 3 - SVDM setup. 1: foam, 2: adhesive film (polyurethane), 3: suction
cup, 4: clip cuts flow, 5: drainage tube, 6: filter, 7 and 10:
connecting tubes, 8: inlet and 9: air outlet, 11: timer display
(digital), 12: start button, 13: vacuum gauge display (pneumatic),
14: vacuum adjustment knob.
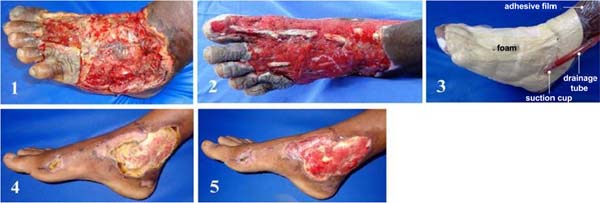
Figure 4 - Example of dressing results used in the management of
contaminated wounds. SVDM: (1) before and (2) after 10 days of
vacuum therapy, (3) SVDM installed; hydrofiber: (4) before and (5)
after 10 days of hydrofiber. Note shorter treatment time and
better-quality granulation tissue with the use of SVDM (cleaning,
development, and color).
Figure 4 - Example of dressing results used in the management of
contaminated wounds. SVDM: (1) before and (2) after 10 days of
vacuum therapy, (3) SVDM installed; hydrofiber: (4) before and (5)
after 10 days of hydrofiber. Note shorter treatment time and
better-quality granulation tissue with the use of SVDM (cleaning,
development, and color).
In both groups, debridements were performed to remove devitalized tissue
occasionally present. Changes were made at ≥ 50% saturation of dressings to
avoid unpleasant odor27.
Patients were followed for 14 days or until the granular lesion (≥ 75% of the
raw bed covered by healthy-looking granulation tissue).
The operational (ease of application and use) and financial feasibility (cost of
dressing changes) of the SVDM were evaluated. For operational feasibility,
outcomes analyzed were application time and amount of dressings; for financial
viability, total economic costs, and cost of dressing changes. Due to the
asymmetry of study variables, statistical analyses were performed using the
median, interquartile range, and bivariate standardized difference to compare
types of dressings.
Difference qualification criteria standardized were: [0-0.2]: absent; (0.2-0.5]:
small; (0.5-0.8]: moderate; [>0.8]: large (Cohen, 1988).
P-values calculated from the same test were adjusted for four
multiple comparisons under dependence conditions by the Benjamini &
Yekutieli method28. For cost
estimates adjusted for dressing application time, number of dressings, and
treatment time, the robust regression model was used with τ = 0.5
(median)29. An overall
α error of 0.05 was assumed for the entire study.
RESULTS
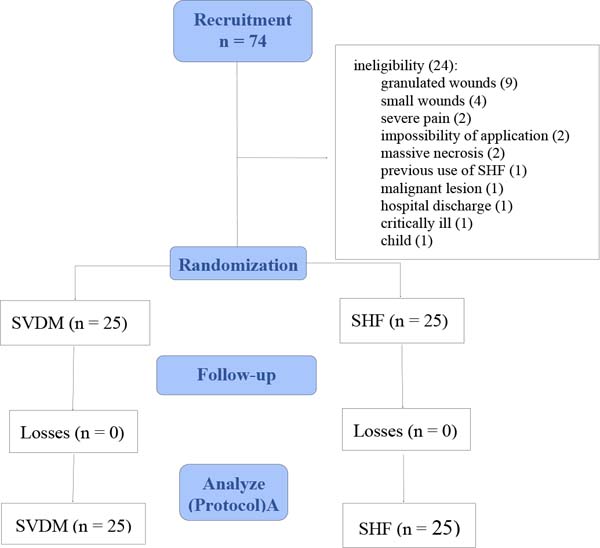
Of the 74 inpatients evaluated, 24 were excluded because they did not meet the
inclusion criteria (Figure 5). Patients
studied were mainly men (SVDM: 52% x SHF: 68%), mixed race (SVDM: 72% x SHF:
84%), non-obese (88%, both groups), and mean age in the age group 6th
decade (SVDM: 55 years x SHF: 50 years -Table 1). Adding the results of both groups, 270 dressings were applied in
589 days of treatment.
Table 1 - Demographic characterization of samples according to groups.
| Variable |
SVDM (n = 25) |
SHF
(n = 25)
|
| Mean (SD)
(CV%)
|
Min/Max |
Mean (SD)
(CV%)
|
Min/Max |
| Age (years) |
55 (14) (25) |
29/85 |
50 (16) (32) |
15/79 |
| Weight
(Kg) |
67 (16)
(23.9)
|
47/108 |
68 (15)
(21.8)
|
43/103 |
| Height (cm) |
164 (11) (6.9) |
145/184 |
166 (12) (6.9) |
154/180 |
|
n |
% |
n |
% |
| Sex |
|
|
|
|
| Men |
13 |
52 |
17 |
68 |
| Women |
12 |
48 |
8 |
32 |
| Ethnicity |
|
|
|
|
| Brown |
18 |
72 |
21 |
84 |
| Black |
5 |
20 |
2 |
8 |
| White |
2 |
8 |
2 |
8 |
| BMI |
|
|
|
|
| Low weight |
2 |
8 |
3 |
12 |
| Normal |
10 |
40 |
11 |
44 |
| Overweight |
10 |
40 |
8 |
32 |
| Obesity |
3 |
12 |
3 |
12 |
Table 1 - Demographic characterization of samples according to groups.
The median application time of the simplified dressing, in minutes, was about 6
times greater than that of SHF (22,7 min x 4,0 min; Sd=0.84;
p=0.0008). SVDM group showed a difference, for less, of 4 days
of treatment (3 days x 7 days; Sd=0.57; p=0.0028) and of 4
dressing changes (3 dressings x 7 dressings; Sd = 0.85;
p<0.0027) (Table 2).
Table 2 - Operational viability according to the type of dressing.
| Variable |
SVDM (n = 25) |
SHF
(n= 25)
|
| Md(IIQ R) |
Min/Max |
CVMd% |
Md(IIQR) |
Min/Max |
CVMd% |
Sd |
p* |
Installation time
of dressing
(min)
|
22.71 (10.0) |
16.5/38.7 |
44.0 |
4.0(3.0) |
2.2/10.4 |
75.6 |
0.84 |
0.0008 |
| Treatment time (days) |
10(5) |
3/15 |
50.0 |
14(0) |
7/15 |
0.0 |
0.57 |
0.0028 |
| Dressings/patient |
3(1) |
1/4 |
33.3 |
7(2) |
6/14 |
28.6 |
0.85 |
0.0027 |
Table 2 - Operational viability according to the type of dressing.
Table 3 (financial feasibility) presents
cost estimates adjusted for application time per dressing, number of dressings,
and treatment time using a robust regression model with τ = 0.5 (median). In
the
raw model (which only contains the type of dressing as an independent variable),
it is observed that the difference in predicted cost of SVDM to SHF was only
US$
5.88. However, when adding other variables mentioned (covariates), the
difference became US$ 209.99 (adjusted model 1). There was, therefore, a
significant change when considering all covariates; thus, the crude model proved
unsatisfactory for predicting the median cost difference between dressings.
Table 3 - Estimating median cost adjusted by dressing application time, number
of dressings, and treatment time using robust regression with τ = 0.5
(median).
| Variable |
Gross
model
|
Adjusted
model 1
|
Adjusted
model 2
|
Saturated
model
|
Final adjusted model |
| Cost (R$) |
pB |
Cost (R$) |
pAj1 |
VIF |
Cost (R$) |
pAj2 |
Cost (R$) |
pS |
Cost (R$) |
pAjf |
| Intercept (β0) |
931.26 |
<
0.0001
|
-1139.05 |
< 0.0001 |
- |
-1269.37 |
< 0.0001 |
-894.75 |
0.1960 |
-1270.55 |
< 0.0001 |
| SVDM
(β1)
|
-31.19 |
0.8470 |
1112.96 |
0.0001 |
2.10 |
1275.15 |
<
0.0001
|
890.53 |
0.1978 |
1282.82 |
<
0.0001
|
| Installation time per dressing (min)
(β2)
|
- |
- |
0.74 |
0.7080 |
2.52 |
0.31 |
0.9138 |
- |
- |
- |
- |
| Number of
dressings (β3)
|
- |
- |
246.00 |
0.0017 |
55.89 |
297.17 |
<
0.0001
|
245.92 |
0.0137 |
297.48 |
<
0.0001
|
| Treatment time (days) (β4)
|
- |
- |
17.03 |
0.4032 |
47.72 |
- |
- |
- |
- |
- |
- |
SVDM
(β1) x
Number of dressings
(β3)
|
- |
- |
- |
- |
- |
- |
- |
58.20 |
0.5462 |
- |
- |
Table 3 - Estimating median cost adjusted by dressing application time, number
of dressings, and treatment time using robust regression with τ = 0.5
(median).
Adjusted model 1 showed a strong correlation (multicollinearity) between the
number of dressings and the treatment
time, with variance inflation factor (VIF) values greater than
ten31. Therefore,
these covariates cannot remain together in the model to avoid bias. As the
number of dressings was the covariate that showed the most
remarkable difference in cost, the covariate, the treatment
time was excluded from the model.
In adjusted model 2, the application time covariate did not
contribute to the predicted cost (US$ 0.31; p = 0.9138), being
removed from the model.
In adjusted model 2, covariates that contributed to median cost estimates were
the type of dressing and the number of dressings. However, their probable
absence was evidenced after evaluating the interaction between these covariates
in a saturated model with an interaction term (p=0.5462).
The final adjusted model shows that the estimated cost difference between SVDM
and SHF was US$ 242.04 (p<0.0001). In other words, costs
would be much higher for SVDM if the group required the same number of dressings
as SHF. Since more SHF dressings were changed (SHF: 7 x SVDM: 3), both the cost
difference found directly in the study (continuous lines) and the difference
if
the groups had the same number of dressings (dashed line) were represented in
Figure 6.
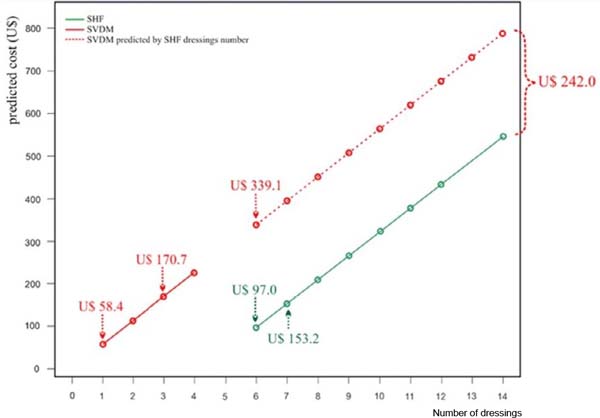
Figure 6 - Predicted cost based on a robust regression model with τ = 0.5
(median) as a function of the number of dressings according to the
type of dressing.
Figure 6 - Predicted cost based on a robust regression model with τ = 0.5
(median) as a function of the number of dressings according to the
type of dressing.
The figure shows, for example, that the estimated costs for six dressings
(minimum number of dressings performed in the SHF group) were: SVDM: US$ 339.09
x SHF: US$ 97.04; estimated costs for the median number of dressings served in
each group were SVDM (3 dressings): US$ 170.70 x SHF (7 dressings): US$ 153.17
(i.e., US$ 17.53 more per patient); finally, the estimated cost for 1 SVDM was
US$ 58.44, which corresponds to an estimated price for 5.31 SHF. Therefore, the
SVDM estimated cost was higher than the SHF estimated cost in all items
evaluated.
DISCUSSION
SVD reduces technological resources to facilitate handling and minimize costs.
However, simplification must not compromise product reliability8,11,16. To ensure
safety, it is recommended that any NPTW equipment has suction control mechanisms
to avoid extreme variations in subatmospheric pressure and, in cases of intense
pressure, to prevent exsanguination through treated wounds3.
SVDM, in addition to containing these safety elements, including a pneumatic
pressure gauge, was equipped with a small specialized filter (high molecular
weight polyethylene - Figure 3) that
becomes impervious when coming into contact with exudates, ensuring blockage
of
effluxes beyond the liquid collection canister. Finally, light-colored foams
were used to facilitate observation of their degree of saturation and retention
of debris. Conventional NPWT uses black foams, which makes this observation
impossible. The transparency of a dressing allows continuous monitoring of wound
beds and perilesional skin without violating the dressing, reducing changes and
costs32.
Few data are available on handling vacuum dressings, making satisfactory
discussions difficult. In a systematic review, the application method was
briefly described without illustrations26. In line with the current monograph, a comparative
study of chronic wound treatment using a wall suction SVD also reported six
steps for applying the vacuum dressing13. Except for these papers and what is recorded in the
conventional NPWT manufacturing manual, descriptions of placement steps have
not
been made in reviews on the subject3,4,33.
The longer application time SVDM (22.7 min x 3.98 min) was attributed to the
greater complexity of using the device and, therefore, the need for training
to
master the procedure. The complexity was due to the multiple steps required for
placement of the SVDM (6 steps x 2 steps), the increased care needed for sealing
dressings (Figure 2, step 2), and the extra
time required to remove foams adhered to wounds.
Only one randomized trial using an SVD model (also powered by the hospital
vacuum) provided results in the references consulted, with an average
application time of 19 min34.
Compared with the present study, the difference of just under four minutes for
dressing changes (22.71 min x 19 min) was considered clinically unimportant.
The
data suggests that the application complexity of the SVDM may be similar to that
of other simplified dressing models.
Application complexity is also a problem related to conventional NPWT1,3,6, especially in
wounds located in contoured areas (e.g., neck, hands, and feet), in places with
recesses (e.g., between fingers, intergluteal crease), in regions that have
natural orifices (e.g., perineum) or when perilesional skin is continuously
moist (dermatoses, dermabrasions, burns, avulsions, among other
conditions)1,3,6,13,35. The complexity is so
significant that conventional NPWT is performed by highly trained nursing teams
who do not work in the hospital that hires them, making it difficult for this
team to access, especially at night, on weekends, in intensive care units, and
operating rooms. Furthermore, obtaining and maintaining wound seals can be a
frustrating exercise, further diminishing the popularity of vacuum
dressings36. In
contrast, occlusive dressings such as SHF are simple, direct, and quick to
apply24. Finally, NPWT
requires the additional work of daily face-to-face monitoring to prevent
leaks13,14.
The SVDM maintained wound seals, controlled subatmospheric pressure, and drained
exudates without early exchanges. As a result, maintenance of the SVDM (3 days)
was similar to that described for most of both standard NPWT or other SVD (2
to
3 days)6,14,37.
Vacuum dressings can be fully functional until ten days if the adhesive film
is
kept intact13,38. There was a reduction in the number of
dressings in the SVDM group (3 x 7), attributed to the continuous drainage of
fluids, which kept dressings unsaturated and operating longer8. Foams used in NPWT, thanks to
their absorbent properties, allow fewer exchanges. To avoid making them smelly
or adhesive, the dressings should be changed every two to three days39,40.
Accumulation of liquid during intermittency can break the film and result in
leaks; consequently, intermittent NPWT has been replaced by a “variable NPWT,”
characterized by a smooth cycle of variation between less intense pressures (-80
mmHg and -10mmHg) to maintain a continuous subatmospheric environment41,42. SVD powered by wall suction, such as the SVDM, may be
desirable, as pressure variations in the hospital network (which are transmitted
to the equipment) mimic the effects of variable NPWT.
Costs analysis is challenging, as available data are poor26 and, contrary to what was
performed in the present trial, described without adjustments for covariates.
SVDM costs depended on the average selling price (SVDM unit: US$ 56.6 x SHF unit
15 cm x 15 cm: US$ 20.5), the number of changes, and the type of dressing.
Results indicate that SVDM implies increased costs per patient (US$ 17.5 more)
and per dressing change, with a single SVDM change (US$ 58.5) equivalent to the
approximate cost of 5 SHF changes. However, four dressings are saved when opting
for SVDM. Suppose the number of SVDM exchanges is similar to that of SHF
exchanges; the cost difference increases (US$ 242.0 - Table 3, Figure 6).
Therefore, care to ensure operational quality is essential so that the median
number of SVDM dressing changes is not exceeded (three shifts); otherwise, the
result would be a considerable increase in the final cost.
Direct costs obtained for SVDM appear much lower than for standard NPWT. The cost
of conventional NPWT was estimated to range from US$ 1,750.0 to US$ 3,450.0
weekly and US$ 1,286.0 to US$ 5,452.0 per patient10,12,26,43. In children, the monthly cost of vacuum therapy was
recently estimated at US$ 1,677/patient44. Treatment can become up to 20 times cheaper with
simplified dressings systems than conventional NPWT10,45. SVD
costs can be as low as US$ 6.4/dressing39, US$ 15.0/day14, or 2% of the average cost of using the VAC
System12.
One reason for lower costs is that hospital vacuum systems, dispensing
specialized devices10, supply
SVD. Another reason is using simple, lower-cost, locally manufactured materials
(foams, polyurethane films, canisters, tubes made of PVC plastic,
etc.)11. Finally, the
current study showed that the cost of SVDM can become even lower if the number
of dressings per patient is minimized. The reduction in exchanges is possible,
as fully functioning NPWT dressings for up to ten days have been
described13,38.
CONCLUSION
SVDM proved greater operational complexity and cost, but it can be feasible as
long professionals for the application master the procedure and there are no
more than three dressing changes per patient.
1. Universidade Federal da Bahia, Salvador, BA,
Brazil
2. Secretaria de Saúde do Estado da Bahia,
Salvador, BA, Brazil
Corresponding author: Sandro Cilindro de Souza Av.
Reitor Miguel Calmon, sala 110, 1º andar, Vale do Canela, Salvador, BA, Brazil.,
Zip Code: 40110-902, E-mail: sandrocilin@gmail.com