INTRODUCTION
Mastopexies are among the most performed aesthetic procedures globally but still have
a high patient dissatisfaction rate, with high rates of secondary surgeries for review
(8-20%)1,2. The difficulties associated with this procedure derive from the antagonistic principle
that the two techniques involved in it exert. The increase, due to tissue expansion,
while mastopexy involves remodeling with excision of parenchyma and skin3.
Several techniques have been described in consideration of those above and the search
for improvement and a lower complication rate. Type of incision, dissection plan,
envelope model are some of the variables in which they are sought as an alternative4-9.
Kahn described a technique with biplanar dissection of the pectoralis major muscle,
creating a superior envelope for prosthesis inclusion in this space. Some of this
modality’s advantages are the transition and smoother contours and more accelerated
postoperative recovery10.
In our clinic, located in Ibitinga, in São Paulo’s interior, we chose to perform this
technique in association with the tactics described by Milton Jaime Daniel, in which
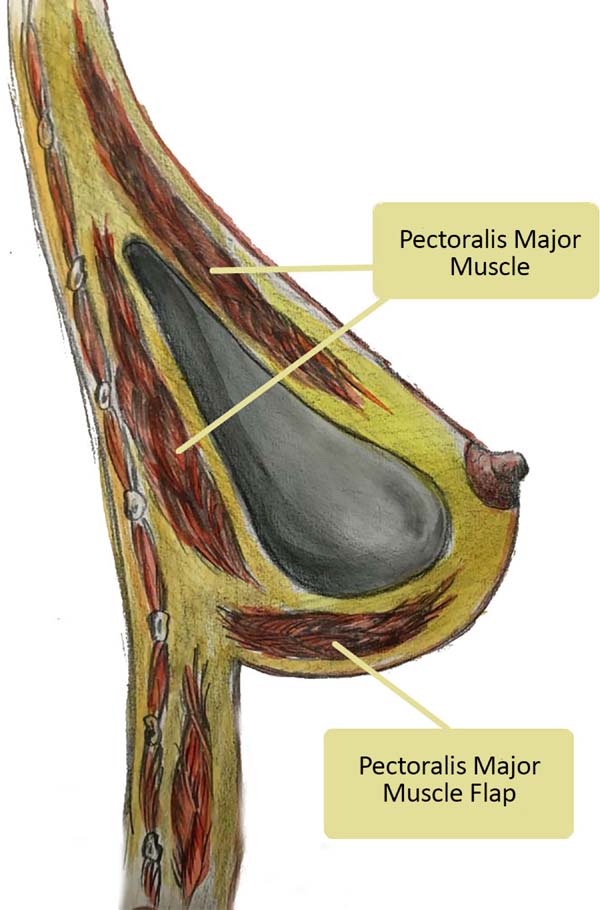
the lower pole is fixed and covered through a lower flap of the pectoralis major muscle11 (Figure 1). A natural aspect is achieved through this combination in the thoraco-mammary transition,
with soft upper pole and maintenance of the breasts’ position and projection.
Figure 1 - Split in the upper region and lower pectoralis muscle brace major.
Figure 1 - Split in the upper region and lower pectoralis muscle brace major.
OBJECTIVES
Describe the surgical technique and evaluate the results of augmentation mastopexies
performed in the author’s private clinic from January 2017 to May 2019.
METHODS
According to Regnault’s Classification, a retrospective study was conducted of patients
with grade II or III mammary ptosis in 197612, operated from January 2017 to May 2019, at the Dr. Jerome Clinic analysis of medical
records. In the preoperative examination, most patients had flaccid skin. According
to the technique described below, all of them were submitted to mastopexy with breast
implants and the association of the lower support flap of the pectoralis major muscle
with a superior split of the same.
This study was conducted according to the Declaration of Helsinki for human studies.
Surgical description
Skin marking
With the patient in an orthostatic position, the thorax’s midline, the breast meridian
and the inframammary groove are defined. The upper edge of the areolas is marked exactly
as described by Pitanguy in 196213, projection of the inframammary groove in the breast meridian, point A. Then points
B and C are determined by digital clamping maneuver, and point D is located in the
previously marked breast groove (Figure 2).
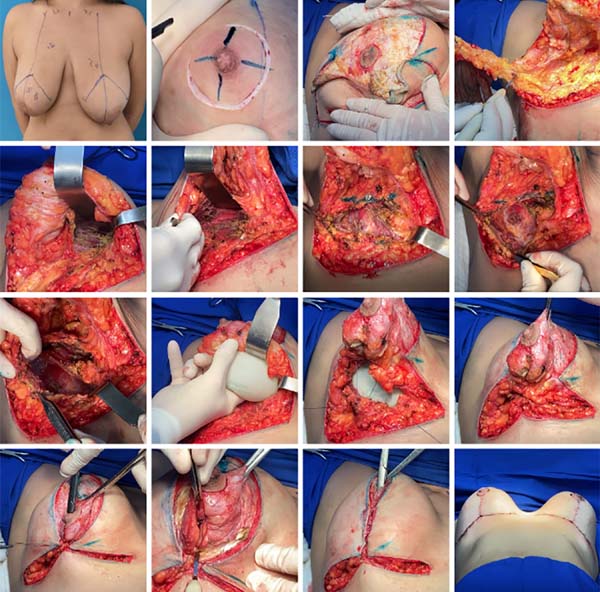
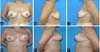
Figure 2 - From preoperative marking to incision, dissection and preparation of the upper and
lower flaps, in addition to the inclusion of implants.
Figure 2 - From preoperative marking to incision, dissection and preparation of the upper and
lower flaps, in addition to the inclusion of implants.
Anesthesia and surgical technique
General anesthesia and infiltration in the breasts with 1% xylocaine solution and
1:200,000 adrenaline are used.
With the patient positioned in the supine position and a slight elevation of the back
(30 degrees), the areolar incision is made with the Schwartzman maneuver, crossing
the region from point A to points B and C. It is made resection in the larger or smaller
keel, depending on breast size and preoperative planning, until reaching the pectoralis
major muscle’s aponeurosis, preserving the lateral and medial pillars using the electric
scalpel.
The upper implant pocket’s preparation is performed through the subfascial plane’s
dissection until the breast’s areolar part. From this location, the pectoralis major
muscle is split, reaching a size close to that of the implant base. The lower pocket
is performed through an incision in the pectoralis major muscle, in the sense of its
fibers, 3 cm from its inferior insertion, towards the sternal and axillary region
(Figure 2).
Rigorous hemostasis and positioning of the prosthesis are performed, where the upper
part will be in the subfascial plane and the lower part in the submuscular plane.
The starting point of breast assembly is performed by joining the lateral abutments
with the pectoralis major muscle. Subsequent stitches are only in breast tissue. Both
were using Vicryl 2-0.
Partial release of the areolar dermis
Cutaneous excess is assessed using digital clamping and tailor tacking using 2-0 nylon.
With these maneuvers, a greater ascension of the nipple-areola complex (NAC) is achieved
(Figure 2). With the evaluation of the shape and symmetry of the breasts, cutaneous excesses
are removed. To prevent the breast scar from becoming too long, lateral and medial
compensations are performed, ending with an inverted T scar. The nipple-areola complex
is repositioned, and it must be located at the apex of the cone and with an approximate
distance of 6cm from the new mammary fold. The dermis suture is made with simple inverted
stitches and on the skin with intradermal stitches using 4-0 monocryl thread. Drains
are not used, and hospital discharge usually occurs 8 hours after the surgery, using
surgical mesh for 30 days.
The dermis suture is done with simple inverted stitches and on the skin with intradermal
stitches using 4-0 monocryl thread.
No drains are used, and hospital discharge usually occurs after 8 hours of completing
the surgery, using surgical mesh for 30 days.
Implants
Round, high-profile silicone implants with polyurethane texture of the Brands Silimed
and Polytech were used to perform the present study.
RESULTS
A total of 192 patients were submitted to mastopexy with an upper split implant and
lower pectoralis muscle fixation (double space) (Table 1). The patients had a mean age of 43 years, ranging from 19 to 68 years. Bmi ranged
from 16-38Kg/m2, with an average value of 23Kg/m2. The average time of the procedure was 150 minutes. The volume of the implants ranged
from 125 to 625ml, with a mean value of 265ml.
Table 1 - Characteristics of patients and complications.
| Characteristics of patients |
|
|
|
| Withdrawal period, d - d |
|
|
Jan/2017 - May/2019 |
| Total patients, n |
192 |
|
192 |
| Primary mastopexy surgery, n (%) |
150 |
100.0% |
150 (78.1 %) |
| Polyurethane Implants (% of Total) |
150 |
100.0% |
150 (78.1 %) |
| Split of superior pectoral muscle and lower muscle brace, n (% of Total) |
150 |
100.0% |
150 (78.1 %) |
| Secondary mastopexy surgery, n (%) |
42 |
28.0% |
(21.9 %) |
| Polyurethane Implants (% of Total) |
42 |
28.0% |
(21.9 %) |
| Split of superior pectoral muscle and lower muscle brace, n (% of Total) |
42 |
28.0% |
42 (21.9 %) |
| Average age (Range), years |
|
|
43 (19 - 68) |
| Average BMI (Range), Kg/M2 |
|
|
23 (16 - 38) |
| Average follow-up period, (Range) months |
|
|
9 ( 1 - 13) |
| Average surgery time, minutes |
|
|
150 |
| Average volume of implants (Range), ML |
|
|
265 (125 - 625) |
| Complications, n (%) |
21 |
14.0% |
21 (10.9 %) |
| Seromas, n (% of Total) |
7 |
4.7% |
7 (3.6 %) |
| Persistent leading to implant removal, n (% of Total) |
3 |
2.0% |
3 (1.6 %) |
| Implants already relocated in a new approach, n (% of Total) |
2 |
1.3% |
2 (1.0 %) |
| Unaesthetic scars requiring new approach, n (% of Total) |
5 |
3.3% |
5 (2.6 %) |
| Scars on the areolar contour, n (% of total) |
2 |
1.3% |
2 (1.0 %) |
| Scars on the medial part of the horizontal scar, n (% of total) |
3 |
2.0% |
3 (1.6 %) |
| Scars readdressed with local anesthesia and sedation after 12 months, n (% of Total) |
5 |
3.3% |
5 (2.6 %) |
| Asymmetries, n (% of Total) |
5 |
3.3% |
5 (2.6 %) |
| Asymmetries already readdressed with muscle part release, n (% of Total) |
3 |
2.0% |
3 (1.6 %) |
| Hematomas, n (% of Total) |
1 |
0.7% |
1 (0.5 %) |
| Infections, n (% of Total) |
0 |
0.0% |
0 (0.0 %) |
| Small suffering of areola with epitheliolisis, n (% of Total) |
3 |
2.0% |
3 (1.6 %) |
| Epitheliolisis already readdressed with local anesthesia, n (% of Total) |
3 |
2.0% |
3 (1.6 %) |
| Skin Necrosis, n (% of Total) |
0 |
0.0% |
0 (0.0 %) |
Table 1 - Characteristics of patients and complications.
Seven patients presented seroma in the postoperative period (3.6%), and in three (1.5%),
the condition persisted for six months even after clinical treatment with compressive
mesh, oral corticosteroids and antibiotic therapy. Thus, we opted for the bilateral
removal of implants. Two patients have already undergone a new procedure with prostheses’
inclusion, with the same technique described here, the same brand and volume as the
previous ones.
There were unaesthetic scars (2.6%) in five cases, two in areolar contour and three
in the horizontal part, which required a new approach under local anesthesia and sedation,
12 months after the first surgery. Three patients presented small sufferings of the
nipple-areola complex (1.5%), with epitheliosis, which was already readdressed, with
scar revision. Breast asymmetries were observed in 5 patients (2.6%). Three underwent
a surgical procedure to release the lower part of the breast’s muscle that presented
the prosthesis in the highest position. One episode of hematoma was described (0.5%)
but without clinical repercussion and need for approach. There were no cases of infection,
contracture or skin necrosis.
Postoperative follow-up ranged from 1 to 13 months, and in all cases, it was noticed
the maintenance of the projection in the upper pole of the breast, with satisfaction
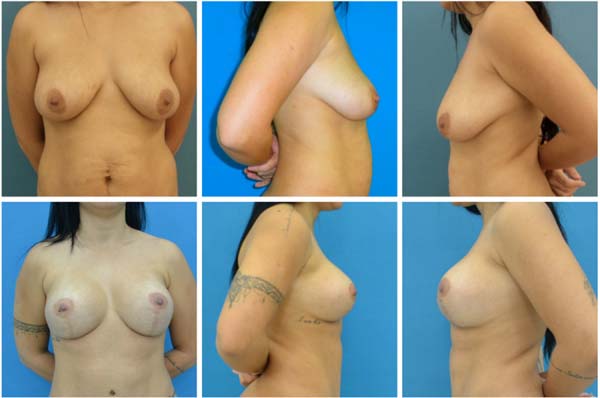
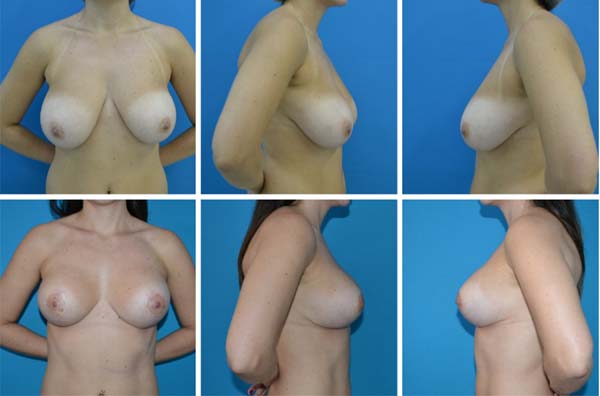
with the aesthetic result, with no recurrence of the breast ptosis (Figures 3 to 6).
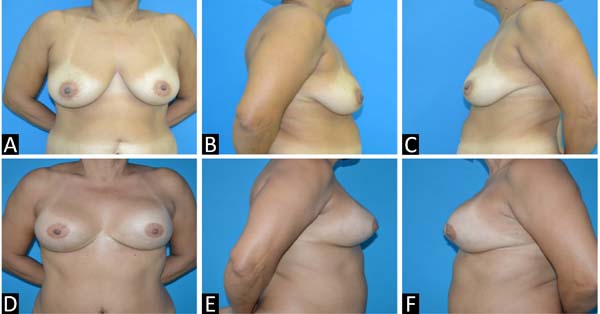
Figure 3 - A, B and C: Preoperative; D, E and F: Postoperative.
Figure 3 - A, B and C: Preoperative; D, E and F: Postoperative.
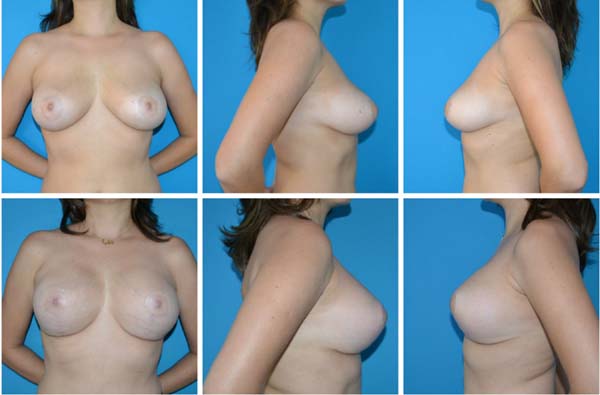
Figure 4 - Pre- and postoperative.
Figure 4 - Pre- and postoperative.
Figure 5 - Pre- and postoperative.
Figure 5 - Pre- and postoperative.
Figure 6 - Pre- and postoperative.
Figure 6 - Pre- and postoperative.
DISCUSSION
Since the original description by Gonzales-Ulloain 196014 and Regnaultin196615, breast augmentation combined with mastopexy remains a challenging and controversial
procedure in plastic surgery, not only for its results but also for its potential
complications16,17.
The smooth transition in the breast’s upper pole and natural appearance made us choose
to make a sandwich of the pectoralis major muscle in its part above the areola, as
recommended by Khan in 200710. This technique provides better coverage of the upper and lateral pole18; another advantage is that there is no dissection of the muscle fibers of its insertion,
preserving function and decreasing the chance of lateralization, displacements of
the prosthesis and deformities of animation19.
In this superior plane, the complications related to the inclusion of the prosthesis
in the total submuscular plane are avoided, such as: displacements, deformities, asymmetries
and rupture; and associated with subglandulars as visible, palpable and rippling prostheses18.
One of the common concerns among the authors is to avoid mammary ptosis. Daniel’s
pectoral muscle flap in 199411provides muscle support in the lower pole of the breast, preventing the implant’s
ptosis and its displacement to the armpit, maintaining the upper pole of the projected
breast. The prosthesis is placed in double space, with its lower pole submuscular
and the rest in the subfascial space. In addition to decreasing the chance of ptosis,
this lower flap offers additional coverage and protection, reducing the chance of
extrusion and exposure of implants20.
We opted for silicone-polyurethane based on studies that suggest that its velcro sticks
to the breast tissues with less displacement of the same within the manufactured pocket
and lower rates of capsular contracture21.
The total number of complications presented, 10.9% of the cases, is lower than that
reported in the literature and the number of secondary surgeries performed - 3.15%.
Asymmetry and seroma were the main causes of new approaches. The first, mainly related
to the lower pedicle preparation, occurred in 2.9% of the patients, but 80% of them
were reoperated. The surgical procedure performed was to enlarge the musculature release
since the complaint was the highest prosthesis concerning the contralateral breast.
Seroma occurred early in 1.5% of patients and remained despite clinical therapy. Secondary
mastopexy was chosen in 100% of them, using the same surgical technique. The other
complications, such as unaesthetic scarring and epidermolysis of NAC, were managed
with small procedures performed under local anesthesia17.
The postoperative evaluations show the effectiveness of this technique, with minimal
ptosis rate, one of the most cited complications in mastopexies. There were no cases
of flattening of the mammary cone, which were more related to periareolar incisions4. The patients were satisfied with the surgical outcome.
The learning curve did not show a hindrance, with an average time of 150 minutes.
The greatest difficulty at the beginning of this technique was dissection for bipartition
of the pectoralis major muscle in its upper part.
The limitations present in this study are the retrospective character and be based
on a single institution and a single surgeon’s experience.
CONCLUSION
The mastopexy with double-space implantation, using a flap of the pectoralis major
muscle in its lower third and the bipartition of the pectoralis muscle in the upper
portion (split pectoralis major muscle), proved to be a reproducible and effective
method in the prevention of post-breast ptosis operation, maintaining the projection
of the upper pole of the breast, in addition to providing greater coverage of the
implant and reducing its exposure risk.
REFERENCES
1. International Society of Aesthetic Plastic Surgery (ISAPS). ISAPS 2017 Global statistics
[Internet]. West Lebanon: ISAPS; 2017; [acesso em 2019 Set 30]. Disponível em: https://www.isaps.org/medical-professionals/isaps-global-statistics/
2. Ismail KT, Ismail MT, Ismail TA, Ismail AT, Toth B. Triple-plane augmentation mastopexy.
Plast Reconstr Surg Glob Open. 2019 Ago;7(8):e2344. DOI: https://doi.org/10.1097/GOX.0000000000002344
3. Sarosiek K, Maxwell GP, Unger JG. Getting the most out of augmentation-mastopexy.
Plast Reconstr Surg. 2018 Nov;142(5):742e-59e. DOI: https://doi.org/10.1097/PRS.0000000000004961
4. Gomes RS. Mastopexia com retalho de pedículo superior e implante de silicone. Rev
Bras Cir Plást. 2008;23(4):241-7.
5. Procópio LD, Silva DDP, Rosique R. Implante submuscular em duplo bolso para mastopexias
de aumento. Rev Bras Cir Plást. 2019;34(2):187-95.
6. Vieira LFDF, Almeida CLA. Mastopexia a Longacre modificada. Rev Bras Cir Plást. 2012;27(1):67-72.
7. Valente DS, Carvalho LA, Zanella RK. Mastopexia crescente com implantes de silicone:
um estudo longitudinal prospectivo. Rev Bras Cir Plást. 2012;27(4):584-7.
8. Pessoa MCM, Accorsi Junior A, Ribeiro L, Moreira LF. Mastopexia com implantes: uso
sistemático dos retalhos de base inferior de Ribeiro. Rev Bras Cir Plást. 2013;28(3):333-42.
9. Tariki JY, Amorim R. Ptose mamária. In: Mélega JM, Viterbo F, Mendes FH, orgs. Cirurgia
plástica: os princípios e a atualidade. Rio de Janeiro: Guanabara Koogan Ltda.; 2011.
v. 1. p. 1166-80.
10. Khan UD. Muscle-splitting breast augmentation: a new pocket in a different plane.
Aesthetic Plast Surg. 2007 Set/Out;31(5):553-8.
11. Daniel MJ. Mamoplastia com retalho de músculo peitoral: uma abordagem dinâmica e definitiva
para a ptose mamária. Arq Catarin Med. 1994;23(Supl 1):37.
12. Regnault P. Breast ptosis: definition and treatment. Clin Plast Surg. 1976 Abr;3(2):193-203.
13. Pitanguy I. Une nouvelle technique de plastie mamaire. Ann Chir Plast. 1962;7:199.
14. Gonzales-Ulloa M. Correction of hypotrophy of the breast by exogenous material. Plast
Reconstr Surg Transp Bull. 1960;25:15-26.
15. Regnault P. The hypoplastic and ptotic breast: a combined operation with prosthetic
augmentation. Plast Reconstr Surg. 1966 Jan;37(1):31-7.
16. Qureshi AA, Myckatyn TM, Tenenbaum MM. Mastopexy and mastopexy-augmentation. Aesthet
Surg J. 2018 Abr;38(4):374-84. DOI: https://doi.org/10.1093/asj/sjx181
17. Artz JD, Tessler O, Clark S, Patel S, Torabi R, Moses M. Can it be safe and aesthetic?
An eight-year retrospective review of mastopexy with concurrent breast augmentation.
Plast Reconstr Surg Glob Open. 2019 Jun;7(6):e2272. DOI: https://doi.org/10.1097/GOX.0000000000002272
18. Rigo MH, Piccinini PS, Sartori LDP, Carvalho LAR, Uebel CO. SMS-Split muscle support:
a reproducible approach for breast implant stabilization. Aesthetic Plast Surg. 2019
Dez;44(3):698-705. DOI: https://doi.org/10.1007/s00266-019-01565-5
19. Siclovan HR, Nistor P. Modified internal mastopexy technique in muscle splitting biplane
breast augmentation. Aesthetic Plast Surg. 2020 Jan;44(3):716-25. DOI: https://doi.org/10.1007/s00266-019-01597-x
20. Stümpfle RL, Piccinini PS, Pereira-Lima LF, Valiati AA. Muscle-splitting augmentation-
mastopexy: implant protection with an inferior dermoglandular flap. Ann Plast Surg.
2019 Fev;82(2):137-44. DOI: https://doi.org/10.1097/SAP.0000000000001689
21. Bozola AR, Bozola AC, Carrazzoni RM. Inclusão de próteses mamárias de silicone poliuretano.
Rev Soc Bras Cir Plást. 2006;21(1):18-22.
1. Clínica Dr. Jerônimo, Plastic Surgery, Ibitinga, SP, Brazil.
2. São Paulo State University (UNESP), Faculty of Medicine of Botucatu, Surgery and
Orthopedics, Botucatu, SP, Brazil.
3. Hospital Ipiranga, Plastic Surgery, São Paulo, SP, Brazil.
Autor correspondente: Jerônimo Martinez Sgarbi, Rua Daniel de Freitas, 972, Centro, Ibitinga, SP, Brazil. Zip Code: 14940-000 E-mail:
jeronimosgarbi@uol.com.br
Article received: August 04, 2020.
Article accepted: January 10, 2021.
Conflicts of interest: none
COLLABORATIONS
JMS Analysis and/or data interpretation, Conception and design study, Data Curation,
Final manuscript approval, Formal Analysis, Realization of operations and/or trials,
Writing - Review & Editing
MSS Analysis and/or data interpretation, Conception and design study, Methodology, Writing
- Original Draft Preparation, Writing - Review & Editing
VS Data Curation