INTRODUCTION
Liposuction is a procedure consecrated in plastic surgery for the improvement of
body contouring. It is the second most performed cosmetic plastic surgery in
the
country1. It was first described for
the treatment of lipodystrophy by French surgeon Illouz in 19832 and has since undergone several technical
developments.
The treatment principle is based on removing subcutaneous tissue (content) from
the regions affected with the preservation of the skin (continent). After vacuum
removal of excess fat, it is assumed that the skin, from its retraction capacity
(elasticity), will adapt to this volume reduction of the subcutaneous tissue.
However, this skin adaptation may not occur, especially in areas with smaller
dermis thickness, such as the thighs and arms’ medial face. This can imply an
unwanted side effect, sagging skin, and removal of excess skin with the creation
of a scar.
In an attempt to solve this issue, different technologies have been developed to
stimulate collagen production and provide more significant skin retraction, such
as local injections of poly-L-lactic acid or calcium hydroxyapatite,
micro-focused ultrasound applications, radiofrequency electrical current
applications, and laser use.
Different laser types can be applied to the subcutaneous tissue and deep dermis
to cause lipolysis and skin retraction3.
The laser with a wavelength of 980nm has an excellent affinity for the water
contained in adipocytes and skin. Therefore, it is effective for lipolysis4 and as a skin retraction stimulator. In
Brazil, this technology has been used for some years. In 2013, Dornelles et al.
5 published a series of 400 patients
submitted to laser lipolysis utilizing this technology and obtaining good
results.
When applied in the subcutaneous and deep dermis region, the laser has a
photochemical (alteration of the adipocyte membrane’s permeability) and
photothermal through heat production, which generates cellular damage to
adipocytes and leads to remodeling of skin collagen 5.
The surgeon who uses this technology is concerned that the production of heat at
the site causes a burn of the tissues. The current parameters (power and energy)
in the devices allow only an indirect inference in skin temperature change.
Therefore, the surgeon needs to choose between applying a laser underdose to
the
tissue without obtaining the expected effect or causing an unwanted burn on
site, without having the precise instruments for this decision making.
Clinical thermography is used in several areas of knowledge currently with
applications in the war industry, construction, and safety. In medicine, the
first article was published in 19576 and
today has several applications such as screening of passengers with infectious
diseases at airports7, quantitative and
qualitative evaluation in the treatment of pain 8, the quantification of brown fat9, which can be used to monitor inflammatory reactions in
orthopedics and rheumatology10,
prevention of injuries in sports medicine11 and for the detection of tumors such as melanomas and their
metastasis12. In plastic surgery, it
has already been used in the preoperative planning of perforating skin flaps,
postoperative follow-up of flaps as a complement in the analysis of the depth
of
burns, in evaluating the hemangioma treatment response, and as a diagnostic test
of carpal tunnel syndrome13. However, to
date, it has not been described to guide laser liposuction procedures.
There are two types of thermographic evaluation: passive and active - also known
as dynamics. In the passive are captured radiometric images with static
measurements of the surface in real-time and at a specific instant. In dynamics,
thermography assesses the temperature of a surface subject to temperature
changes. It measures dynamic physiological responses to stimuli, such as cold
and heat, documented over a time interval. Dynamic evaluation is used in
provocative tests such as brachial occlusion to evaluate the endothelium14, or the so-called cold stress test or
cold challenge test, which activates the autonomic nervous system through
exposure to environmental cold or specific limbs, applied in cases of chronic
pain and neurological evaluation15,16.
OBJECTIVES
This work aims to describe a new standardized dynamic thermography technique to
determine the epidermis temperature in real-time during laser application and,
consequently, guide the surgeon, allowing a thermo-guided procedure, increasing
efficiency, and reducing the risk of complications.
A second objective is to use this standardization to determine the
upper-temperature limit with the method, through the temperature that, on
average, burns on the skin during laser application.
METHODS
Eighty-three patients were submitted to laserlipolysis with the
Orlight® Duo 980nm diode laser (Figure 1), between July 2017 and June 2019, at the Hospital
da Plástica, São Paulo/SP. All patients in the study signed the consent form,
and the study received approval from the ethics committee under number
0126/2019. The age range of the patients ranged from 17 to 75 years. All
patients were female.
Figure 1 - Orlight diode laser® Duo 980nm.
Figure 1 - Orlight diode laser® Duo 980nm.
For temperature monitoring, a Flirthermocamera was used ® model
T540sc (Flir Systems Inc, Wilsonville, OR) with IR resolution of 464x248 pixels
and thermal sensitivity of 30mk and a lens of 42º (Figure 2) fixed to the surgical focus to allow its manipulation by
the surgeon himself in a sterile manner (Figure 3). Bluetooth transmitted the images of the thermocamera to an Ipad
Pro 12.9’’ tablet fixed in front position to the surgeon (Figure 4).
Figure 2 - Thermocamera Flir® model T540sc (Flir Systems Inc,
Wilsonville, OR).
Figure 2 - Thermocamera Flir® model T540sc (Flir Systems Inc,
Wilsonville, OR).
Figure 3 - Fixation of the camera in the surgical focus.
Figure 3 - Fixation of the camera in the surgical focus.
Figure 4 - Tablet with Bluetooth transmission of the images of the
thermocamera, in front position to the surgeon.
Figure 4 - Tablet with Bluetooth transmission of the images of the
thermocamera, in front position to the surgeon.
The temperature and humidity environment (always less than 60%) was controlled
with an HTC-1 thermohygrometer ensuring the patient’s thermal comfort who had
exposed only the body area to be operated. The patients were not exposed to air
currents, and the temperature was maintained at 22ºC.
An alarm was set in the thermocamera when the skin temperature reached 38ºC.
Laser application was used to cause skin retraction was made with the tip
directed against the deep dermis at the linear 5 centimeters speed every 1
second.
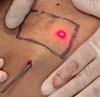
The laser was applied to the tissue until a homogeneous temperature surface was
obtained, observed in the thermocamera as a red color throughout the demarcated
area (Figure 5).
Figure 5 - Demarcated area presenting a homogeneous red color.
Figure 5 - Demarcated area presenting a homogeneous red color.
The laser was applied to the deep dermis of abdominal flaps of patients
undergoing dermolipectomy to determine the temperature that causes skin burns,
totaling 27 cases. According to a previously standardized back table technique,
the temperature was measured immediately after resectioning the abdominal flaps,
with marked areas of 6x6cm bilaterally (Figure 6).
Figure 6 - Prior marking of abdominal flap areas to be tested for burn after
resection of classical dermolipectomy.
Figure 6 - Prior marking of abdominal flap areas to be tested for burn after
resection of classical dermolipectomy.
The skin’s burn temperature was defined as the exact moment of the appearance of
an opaque plaque on the surface of the skin, followed by a defect of a total
thickness (Figure 7).
Figure 7 - Determining the burn temperature with the laser in the flaps of
dermolipectomy in back table.
Figure 7 - Determining the burn temperature with the laser in the flaps of
dermolipectomy in back table.
RESULTS
It was applied in the arm and thigh’s medial face, 15W, and an average of 4000J
of energy per region. In the dorsal and abdomen area, 20W was adopted, and 6000J
of energy was used in each quadrant.
In the subment region, 10W was adopted, and an average of 1500J of energy was
applied (Table 1).
Table 1 - Distribution of power and energy applied, according to the
region.
| Region |
Power (w) |
Energy |
| Medial arm face |
15 |
4000 |
| Medial thigh face |
15 |
4000 |
| Back |
20 |
6000 |
| Abdomen |
20 |
6000 |
| Subment |
10 |
1500 |
Table 1 - Distribution of power and energy applied, according to the
region.
The initial temperature of the studied tissue was, on average, 31ºC, with the
temperature of the room standardized at 22ºC. The average temperature at the
end
of laser application was 37ºC (Table 2).
Table 2 - Average skin temperature.
| Average Temperature (ºC) |
| Skin (initial) |
31 |
| Skin (final) |
37 |
| Burn |
45 |
| Security Interval |
36 to 40 |
Table 2 - Average skin temperature.
The average temperature that caused the burn was 45ºC, and the safety interval in
the thermoguided technique was fixed between 36 and 40º C, adopted as the goal
of laser application.
Nine cases with seroma and the need for punctures in the office were verified,
and two patients presented minor burns in the subment region, treated
conservatively.
DISCUSSION
The generation of heat in the deep dermis region promotes collagen production,
activation of the healing process, contraction of collagen, and thickening its
fibers17. For this effect to occur
correctly, heat must cause intra- and intermolecular rupture of collagen
fibrils, which only occurs within a certain temperature range18. Previous studies have shown that this
temperature ranges from 60 to 65c19.
Various technologies use the principle of temperature increase to induce this
biological effect, such as radiofrequency, ultrasound, and laser20. However, excessive heat can cause
burns. According to Lawrence and Bull, in 197621, an object in contact with the skin for any time duration should
not exceed 42°C temperature because, at temperatures above 43.5°C, there is
tissue damage. Therefore, it is essential to monitor the temperature in the
epidermis so that it remains within a safe range, and at the same time, heat
induces remodeling of the dermis. Among the different methods used for this
purpose, infrared thermography stands out.
Infrared medical thermography is a non-invasive and non-radioactive method of
analysis, capable of monitoring the epidermis’ temperature without contact. In
1964, the first record of contactless thermography in evaluating skin burns with
gradients up to 0.1ºC was published, allowing the estimating of relative
perfusion22. In theory, infrared
radiation emitted from a burn wound should decrease according to increased burn
depth due to the higher degree of microvascular coagulation, and, consequently,
thermography was also applied to calculate the depth of the lesion23,24.
However, static or passive thermography is subject to several factors that can
negatively impact the intratemporal result, such as evaporative heat or heating
and cooling effects, also wound granulation that modifies the level of perfusion
or even emissivity, as well as variations in the depth of vascularization in
the
cutaneous tissue in different places of the body25.
New dynamic thermography techniques were proposed, where thermal energy was
introduced into the skin, raising its physiological temperature. Following the
skin temperature in the lesion in time, regional variations in heat energy
transfer can be detected on the wound surface, allowing the identification of
the burn depth26,27.
In dynamic thermography for the evaluation of deep burns, the temperature
response is evaluated after a thermal pulse excitation: initially, the fixed
temperature distribution on the surface of interest is measured with an infrared
camera; in the sequence is applied the external thermal excitation, following
by
various temperature measurements on the tested surface for a specific time;
finally, the depth of the skin burn can be evaluated quantitatively by
calculating the constant T of the thermal time26.
Thermal imaging shows surface skin burns an increase in temperature compared to
the nearby healthy area, while deep burns have lower temperatures than nearby
healthy regions. This temperature difference is statistically significant and
provides a way to distinguish superficial skin burns from deep burns28.
In this research, we used a thermography camera, which transmits the infrared
image in real-time to Bluetooth’s monitor. Allows thermal monitoring of the
entire treated region, unlike a thermometer that only one specific point at a
time. To have a comparison parameter, when using an infrared camera with a
resolution of 464×348 pixels, this is equivalent to 161,472 measurements
with surface thermometer17.
The laser technique added 3 to 10% of the procedure’s previous total value,
presenting a reasonable cost versus benefit ratio.
The laser’s application in subcutaneous and deep dermis guided by the
thermocamera allowed a more homogeneous energy application in the demarcated
area and provided effective temperature monitoring. The surgeon obtained the
desired effect without reaching the risk temperature for burns.
In this dynamic thermography article, the objective is not to evaluate an
existing lesion or to measure recovery in the proposed technique. The aim was
to
avoid a possible internal deep burn injury, a proactive and preventive action
through a thermoguided laser technique. However, considering the existence of
dynamic, provocative tests in the evaluation of deep burns, this technique can
be improved in future research accompanying patients submitted to this technique
after the procedure to measure possible damage such as deep burns and better
adjust the desired temperature with the laser.
CONCLUSION
Infrared skin thermography is a proper complementary method, non-invasive,
without side effects, whose use during the lipolaser procedure provides reliable
thermal monitoring, thus being an extra parameter that can avoid unwanted deep
burns.
Lipolaser thermocamera monitoring also allowed a more homogeneous distribution of
heat in the treated area. It provided the surgeon with safe monitoring to apply
to the subcutaneous and deep dermis an adequate energy dose to obtain the
desired effect.
Monitoring to the point of burning the skin with laser defines what temperature
this technique offers risk and allows the surgeon to determine the target
temperature he wants to reach in each body area.
Future longitudinal studies are suggested in which the potentials of cutaneous
infrared thermography in the monitoring of the lipolaser process and its
evaluation of the existence of deep subcutaneous burns with provocative thermal
tests are suggested.
ACKNOWLEDGMENT
Thanks to Marcelo Kikuchi, from Laser Medical, for the support and supply of the
equipment.
REFERENCES
1. Sociedade Brasileira de Cirurgia Plástica (SBCP). Censo 2016.
Situação da Cirurgia Plástica no Brasil. Análise comparativa das pesquisas 2014
e 2016. São Paulo: SBCP; 2017; [acesso em 2019 Jun 15]. Disponível em: http://www2.cirurgiaplastica.org.br/wp-content/uploads/2017/12/CENSO-2017.pdf
2. Illouz YG. Body contouring by lipolisis: a 5-year experience with
over 3000 cases. Plast Recons Surg. 1983 Nov;72(5):591-7.
3. Weiss RA, Beasley K. Laser assisted liposuction using a novel blend
of lipid and water-selective wavelenghts. Lasers Surg Med. 2009
Dez;41(10):760-6.
4. Centurion P, Cuba JL, Noriega A. Lipossucción con diodo láser 980nm:
optimización de protocolo seguro em cirurgía de contorno corporal. Cir Plást
Iberolatinoam. 2011 Jun;37(4):355-64.
5. Dornelles RFV, Lima e Silva A, Missel J, Centurion P. Laserlipólise
com diodo 980nm: experiência com 400 casos. Rev Bras Cir Plást.
2013;28(1):124-9.
6. Lawson R. Implications of surface temperatures in the diagnosis of
breast cancer. Can Med Assoc J. 1956 Jun;75(4):309-10.
7. Chan LS, Lo JLF, Kumana CR, Cheung BMY. Utility of infrared
thermography for screening febrile subjects. Hong Kong Med J. 2013
Apr;19(2):109-15.
8. Nahm FS. Infrared thermography in pain medicine. Korean J Pain. 2013
Jul;26(3):219-22.
9. Ang QY, Goh HJ, Cao Y, Li Y, Chan SP, Swain JL, et al. A new method
of infrared thermography for quantification of brown adipose tissue activation
in healthy adults: a randomized trial. J Physiol Sci. 2017
Mai;67(3):395-406.
10. Engel JM, Saier U. Thermographische standarduntersuchungen in der
rheumatologie und richtlinien zu deren befundung. Baden-Baden: Verlag;
1984.
11. Moreira DG, Costello JT, Brito CJ, Adamczyk JG, Ammer K, Bach AJ, et
al. Thermographic imaging in sports and exercise medicine: a Delphi study and
consensus statement on the measurement of human skin temperature. J Thermal
Biol. 2017 Out;69:155-62.
12. Herman C. The role of dynamic infrared imaging in melanoma
diagnosis. Expert Rev Dermatol. 2013 Apr;8(2):177-84.
13. John HE, Niumsawatt V, Rozen WM, Whitaker IS. Clinical applications
of dynamic infrared thermography in plastic surgery: a systematic review. Gland
Surg. 2016 Abr;5(2):12-32.
14. Ley O, Deshpande C, Prapamcham B. Lumped parameter thermal model for
the study of vascular reactivity in the fingertip. J Biomech Eng. 2008
Mai;130(3):031012.
15. Davey M, Eglin C, House J, Tipton M. The contribution of blood flow
to the skin temperature responses during a cold sensitivity test. Eur J Appl
Physiol. 2013 Set;113(9):2411-7.
16. Suzuki Y, Kobayashi M, Kuwabara K, Kawabe M, Kikuchi C, Fukuda M.
Skin temperature responses to cold stress in patients with severe motor and
intellectual disabilities. Brain Develop. 2013 Mar;35(3):265-9.
17. Key DJ. Integration of thermal imaging with surface radiofrequency
thermistor heating. J Drugs Dermatol. 2014 Dez;13(12):1485-9.
18. Sadick N. Tissue tightening technologies. Aesthet Surg J. 2008
Mar;28(2):180-8.
19. Hayashi K, Thabit G, Massa KL, Bogdanske JJ, Cooley AJ, Orwin JF, et
al. The effect of thermal heating on the length and histologic properties of
the
glenohumeral joint capsule. Am J Sports Med. 1997
Jan/Fev;25(1):107-12.
20. Pritzker RN, Hamilton HK, Dover JS. Comparison of different
technologies for noninvasive skin tightening. J Cosmet Dermatol. 2014
Dez;13(4):315-23.
21. Lawrence JC, Bull JP. Thermal conditions which cause skin burns. Q
IMechE. 1976;5(3):61-3.
22. Lawson RN, Gaston JP. Temperature measurements of localized
pathological processes. Ann N Y Acad Sci. 1964 Out;121:90-8.
23. Mladick R, Goergiade N, Thorne F. A clinical evaluation of the use
of thermography in determining degree of burn injury. Plast Reconstr Surg. 1966
Dez;38(6):512-8.
24. Watson AC, Vasilescu C. Thermography in plastic surgery. J R Coll
Surg Edinb. 1972 Jun;17(4):247-52.
25. Anselmo V, Zawacki B. Infra-red photography as a diagnostic tool for
the burn ward. Proc Soc Photo Opt Instr Eng. 1973;8:181.
26. Renkielska A, Nowakowski A, Kaczmarek M, Ruminksi J. Burn depths
evaluation based on active dynamic IR thermal imaging--a preliminary study.
Burns. 2006 Nov;32(7):867-75.
27. Prindeze NJ, Fathi P, Mino MJ, Mauskar NA, Travis TE, Paul DW, et
al. Examination of the early diagnostic applicability of active dynamic
thermography for burn wound depth assessment and concept analysis. J Burn Care
Res. 2015 Nov/Dez;36(6):626-35.
28. Medina-Preciado JD, Kolosovas-Machuca ES, Velez-Gomez E,
Miranda-Altamirano A, González FJ. Noninvasive determination of burn depth in
children by digital infrared thermal imaging. J Biomed Optics.
2013;18(6):061204.
1. Hospital da Plástica SP, Department of Plastic
Surgery, São Paulo, SP, Brazil.
2. Kamamoto Clinic, Plastic Surgery, São Paulo,
SP, Brazil.
3. Poliscan Brasil, Medical equipment, São Paulo,
SP, Brazil.
Corresponding author: Fabio Kamamoto,
Rua Mato Grosso, Higienópolis, 306, Conjunto 1402, São Paulo, SP, Brazil. Zip
Code: 01239-040. E-mail: fabio.kamamoto@gmail.com
Article received: November 21, 2019.
Article accepted: October 22, 2020.
Conflicts of interest: none