INTRODUCTION
The use of autologous fat grafting to correct defects was first described over 100
years ago when it was used to correct facial defects. Concurrently a back lipoma was
also used to recreate a patient’s breast after mastectomy1.
The contemporary evolution of autologous fat grafting was popularized by Coleman et
al., in 20102, who described the use of liposuction and adipocyte purification for injecting into
the face as a soft tissue filling. Then, Bircoll and Novack (1987 apud Costantini
et al.), extended this use to breasts3,4.
The interest in fat injection was revived in the early 1990s by Coleman, who confirmed
that adipose tissue could be satisfactorily transferred by stipulating that a strict
protocol for fat preparation and injection should be respected2,5.
Coleman’s fat grafting technique is by far the most commonly used. The adipose tissue
is infiltrated with a tumescent solution (for example, Klein’s solution) and then
manually harvested through skin incisions using a 3 mm cannula with two holes and
blunt tip connected to a 10 mL syringe. The liposuction material is subsequently centrifuged
for three min at 3,000 rpm to isolate the adipose tissue from the oily and aqueous
fraction and then injected. The entire procedure can be performed under local anesthesia2,6.
The analysis of different anesthetic drugs showed greater viability of adipose stem
cells in adipose tissue treated with bupivacaine, mepivacaine, ropivacaine, and lidocaine
compared to the combined treatment with articaine and epinephrine. Although no variability
between amides is expected, epinephrine can affect α1 receptors in adjacent tissues
that support the implanted cells. In general, the tumescent solution improved cell
viability compared to the dry technique. No significant differences were observed
between the commonly used anesthetics, except for articaine and epinephrine7.
Transferring fat from an excess area such as the abdomen or thighs to improve the
shape and volume of the breast is not a new idea. A study by Illouz, in 19838, promoted the widespread use of liposuction worldwide, making the use of fat from
adipose deposits to increase breast volume.
The injected amounts varied from 100 to 250 mL in each breast6. In 20009, Fournier carefully affirmed that the fat was only injected in the retro glandular
space and not in the mammary parenchyma.
Fat grafting has also been used to treat burn scars. The evolution of the scarring
one year after treatment was evaluated using a questionnaire as well as physical and
histopathological exams. In the first year of follow-up, all patients reported improved
clinical condition. Histological findings showed new collagen deposition, neoangiogenesis,
and dermal hyperplasia in the context of new tissue, demonstrating tissue regeneration10.
Postoperative mammographic images to control fat necrosis vary. These can present
as lipid cysts, suspected malignant findings such as grouped microcalcifications,
spiculated areas of increased opacity, and focal masses10.
Fat necrosis is a nonspecific histological finding that involves several processes
in its etiopathogenesis. In addition to surgery, the most common causes of fat necrosis
are ischemia, radiation therapy, and trauma. There are reports of some other rare
occurrences of breast fat necrosis caused by anticoagulant therapy with sodium warfarin
(Coumadin) and sodium enoxaparin. Calciphylaxis, which is hypersensitivity to local
calcinosis associated with secondary hyperparathyroidism in renal failure, has also
been reported10.
An increased incidence of cancer after fat grafting was reported in reviews of studies
with animals, proving this hypothesis, but there was no evidence of this in in vitro studies11.
OBJECTIVE
To conduct a literature review on the use of fat grafting in breast reconstructions
with expanders and implants, and report three cases of patients undergoing the procedure
in a private clinic in Fortaleza.
METHODS
A bibliographic review was conducted using the scientific research databases, BIREME,
NCBI, PubMed, and SciELO, as well as on studies published in the journals of the Brazilian
and American Society of Plastic Surgery.
Three cases of patients who underwent breast reconstruction using prostheses and expanders
associated with fat grafting will be described. The incisions were made in the fat
donor areas using a No. 15 scalpel blade. Abdominal fat was aspirated through two
lateral incisions in the flanks using a 3 mm cannula with three holes connected to
a 60 mL syringe.
The fat was injected in small amounts in the shape of thin cylinders by retro-infusion.
It was necessary to create micro-channels in many directions. The transfer was made
from a deep to a superficial plane. Good spatial visualization was necessary to form
a kind of three-dimensional honeycomb to avoid fat pockets forming that would lead
to fat necrosis.
The fat graft was prepared using the sedimentation method. Fat processing is necessary
because the liposuction material contains not only adipocytes but also collagen fibers,
blood, and debris.
The preoperative evaluation was based on anthropometric methods, and the patients
were followed up monthly with a breast ultrasound to control fat grafting and mammography
according to age.
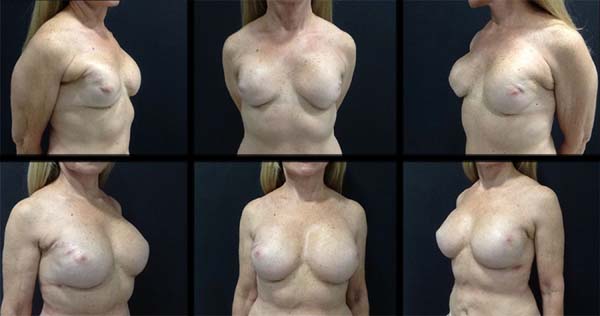
Case 1: Female, 65 years old, married, underwent a total right mastectomy in 2002 and total
left mastectomy in 2008, with immediate reconstruction with 200 mL and 250 mL prostheses,
respectively. The patient returned in 2016 to correct breast asymmetry. Personal morbid
history: nipple-areola complex radiotherapy and hormone therapy for five years. On
examination: asymmetric breasts with grade one ptosis with a bilateral contracture
(Baker 3), which was more intense on the right side and absence of fat tissue bilaterally.
The prostheses were removed and new ones with 332 mL and 350 mL were placed on the
right and left breasts, respectively, in addition to fat grafting of 80 mL on the
right and 60 mL on the left. Second fat grafting was completed two months later using
70 mL on the right and 80 mL on the left (Figure 1).
Figure 1 - Clinical case no. 1.
Figure 1 - Clinical case no. 1.
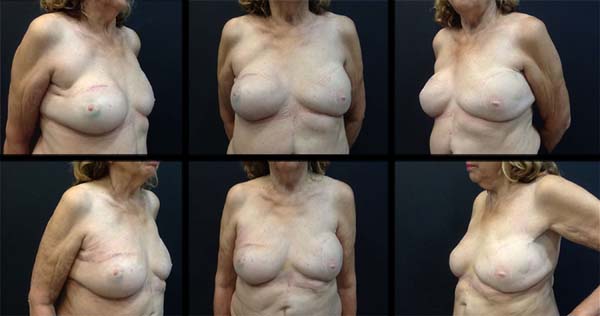
Case 2: Female, 75 years old, married, underwent total right mastectomy due to in situ ductal carcinoma, with a late reconstruction with transverse rectus abdominis myocutaneous
(TRAM) flap in 2007. The patient sought the service due to dissatisfaction with the
obtained result. Personal morbid history: mother with lung and breast melanoma. A
350 mL expander prosthesis was placed on the right breast in December 2015. In June
2016, the right expander was replaced by a 350 mL silicone prosthesis and the left
by a 332 mL prosthesis, in addition to 120 mL fat grafting in the breasts (Figure 2).
Figure 2 - Clinical case no. 2.
Figure 2 - Clinical case no. 2.
Case 3: Female, 38 years old, married, underwent total mastectomy with right axillary emptying
due to tumor > 5cm and immediate reconstruction with a 450 mL expander prosthesis
in 2013, sought the service for breast symmetrization. Personal morbid history: radiotherapy
and neoadjuvant chemotherapy in May 2013. In 2015, the expanders were replaced by
silicone prosthesis of 495 mL on the right and 250 mL on the left breast (Figure 3).
Figure 3 - Clinical case no. 3.
Figure 3 - Clinical case no. 3.
DISCUSSION
The ideal augmentation material requires certain qualities, including biocompatibility,
lack of toxicity, produces consistent and reproducible results, and is cost-effective.
Autologous fat grafts have many of these qualities and also provide a natural feeling,
can be personalized for each patient and easily removed in case of complications or
dissatisfaction. The biggest challenge in autologous fat transfer is to maintain the
longevity and durability of fat grafts. This is related to the fat collection and
preparation techniques. Although there are some clinical studies on this subject,
some important questions have not been answered: (1) Can any current fat grafting
method be considered standard?; (2) Is there a more viable and functional fat preparation
method?; and, (3) Can a common fat collection and preparation protocol be found in
the light of current information in the literature?
In 20072, Coleman et al. questioned a restriction on the use of fat grafting issued by a committee
of American experts in 1987, stating that calcifications and liponecrosis observed
after fat grafting procedures are also observed in other breast procedures, such as
breast reduction and mastopexy.
Many preparation techniques have been suggested in the literature to maintain the
viability of fat grafts after being harvested and processed.
A survey by the American Society for Aesthetic Plastic Surgery reports that Coleman’s
microcannula technique is the most common method of autologous fat collection (54%),
followed by standard liposuction cannula (25%), syringe and large needle caliber (16%),
and direct excision techniques (5%). The same survey found that, after collecting
fat, 47% of respondents perform fat centrifugation, 29% perform fat washing, 12% cite
“other” unspecified treatment techniques, and 12% use no preparation method12.
Other fat collection and processing methods have been reported in the literature and
used clinically for structural fat grafting. Har-Shai et al., in 199913, used an integrated approach in which the fat grafts were harvested with a syringe
and centrifuged at 1,000 rpm. After centrifuging, the aspirate was suspended in an
enriched cell culture medium to increase the survival of autologous fat grafts. They
used this integrated technique in 15 patients and reported that the amount of graft
absorbed varied between 50% and 90% over a follow-up period of between six and 24
months12,14.
Pu et al., in 200815, reported a modified technique that included low-pressure fat aspiration using a
20 mL syringe and separation of adipose tissues using gravity without centrifugation.
Additionally, they refined fat packs with gauze and cotton sticks to remove the greasy
and non-greasy components. Although they reported that a good volume of fat was maintained
in a long-term follow-up, it is important to avoid exposing fat grafts to air to maximize
graft viability and minimize contamination.
They also compared their collection technique with conventional liposuction and showed
that low-pressure fat collection using a syringe produces more adipocytes with optimal
cellular function than the negative pressures greater than 20 cm H2O generated by conventional liposuction. However, there are no comparative studies12,13.
As for use, fat grafting in the chest wall and breast deformities is expanding rapidly.
They seem to represent a significant advance in the treatment of Poland’s syndrome
and will probably revolutionize the treatment of severe cases, producing unparalleled
quality reconstruction with short and straightforward postoperative care and less
scarring5.
This same reasoning is used for grafts in tissue that underwent radiotherapy, in which
vascularization is scarce and, even so, presents good results. Most of the studies
report a high number of complications, with fatty necrosis and images related to it
being the most frequent complication in the radiological follow-up of the grafted
area11.
The percentage of patients requiring another fat grafting session showed no significant
differences between groups. So far, clinical outcomes, fat necrosis in breast ultrasound,
and the need for new fat grafts have demonstrated that fat enriched in platelet-rich
plasma is not superior to fat grafting alone11.
Some positive effects of platelet-rich plasma in angiogenesis and stem cell proliferation
derived from fat tissue have been experimentally demonstrated. As for angiogenesis,
platelet-rich plasma growth factors stimulate endothelial cells around the injection
site, favoring the proliferation and formation of new capillaries. Besides, an in vitro study reported that plasma is a potential contributor in initiating the angiogenesis
process, recruiting endothelial cells that line blood vessels and initiating bone
regeneration13,15,16,17.
As for cell proliferation, activated platelet-rich plasma contains large amounts of
PDGF-AB and TGF-β1 and promoted the proliferation of human stem cells derived from
fat tissue and human dermal fibroblasts in vitro. Cell proliferation was maximum when 5% activated platelet-rich plasma was added
to the culture medium. Paradoxically, the addition of 20% activated platelet-rich
plasma did not show the same results15,16,17.
Lipofilling procedures can modify radiological imaging. However, their interference
has been studied in the literature, and radiological studies suggest that imaging
technologies (ultrasound, mammography, and magnetic resonance imaging) can identify
microcalcifications caused by fat injections. Also, recent follow-up studies have
demonstrated the safety of the procedure, with no increased rates of a new disease
or tumor recurrence being reported18,19.
Oncological follow-ups showed no increased risk of local recurrence after mastectomy
or conservative treatment. The clinical impression seems to suggest the opposite,
but to confirm this, more complex oncological studies comparing populations treated
with reference populations with the same oncological status are necessary5,18,19.
The volume of grafted fat was stable after three to four months and remained constant.
If a patient loses weight, the volume of transferred fat decreases, and the resulting
smaller breast size can lead to asymmetry. Therefore, it is important for patients
to understand the need to maintain a stable weight. Contrastingly, if a patient gains
weight, breast volume increases due to the increased fat deposits.
Reabsorption seemed to be less intense (between 20 to 30%) when a second session was
needed to obtain sufficient volume. This reduced-fat reabsorption rate has been clinically
evaluated. In some cases in which the patients required a second fat transfer session,
an interferometric evaluation objectively confirmed this clinical impression.
Latissimus dorsi flaps have gradually replaced TRAM flaps in the last ten years due
to more straightforward postoperative care and better use of local thoracic tissue,
avoiding a patch effect on the breast. However, in some cases, if the patient is very
thin or if there is severe flap atrophy, the reconstructed breast can be very small.
Autologous latissimus dorsal flap is the most suitable tissue to receive a fat transfer,
as it is very well vascularized, and large amounts of fat can be injected. In the
early stages of this experiment, moderate amounts (100 to 120 mL) were injected but
due to the reabsorption rate, this quantity was not enough. Lipomodelling made it
possible to correct localized abnormalities or defects in the neckline6.
Some techniques to evaluate breast volume are described in the literature for historical
purposes, and others are modern, practical, and reliable.
1. Anthropometric method
Based on end-to-end measurements, the female breast is geometrically visualized as
a half ellipse, and its volume can be calculated using mathematical formulas. Measurements
can be performed using photographs or mammograms. It is a practical technique, but
it depends on the proficiency of the examiner20.
2. The Grossman-Roudner measuring device
It is a circular plastic device with a cut along the radius line. It proved to be
practical and very profitable, as the cost of time and material total only 1.00 USD.
Although it has been used for anthropomorphic measurements of the breast in 50 women,
there is no precise validation for this method20.
3. Archimedes’ principle
A simple method of historical value based on the Archimedes’ principle of water displacement20.
4. 3D surface image
The use of 3D imaging devices provides a virtual 3D model of a standing patient that
facilitates the elimination of breast tissue compression. It simulates the post-augmentation
status and can help patients to define their desired augmentation volume. It is a
noninvasive method, and data collection does not depend on the examiner when it is
based on standardized protocols. Since magnetic resonance imaging (MRI) is considered
the gold standard for non-contact volume measurements, the validity of the 3D image
was compared to MR measurements20.
5. Volume measurement with nuclear MR
Based on a high sensitivity to detect complications in autologous fat transplantation,
such as oil cysts, calcifications, or necrosis, MRI has great importance after autologous
fat transplantation to the breast. MRI is already considered the gold standard in
detecting other breast pathologies such as implant ruptures, and its use in breast
imaging is increasing. However, in addition to qualitative assessment, MRI scans of
the breast can also be used for quantitative assessment20.
RESULTS
Case 1. After six years of primary breast reconstruction, the patient presented with a bilateral
capsular contracture due to post-adjuvant radiotherapy classified as Baker 3. New
332 mL and 350 mL prostheses were placed on the right and left breasts, respectively,
with fat grafting of 80 mL on the right and 60 mL on the left breasts, showing a good
breast envelope. After two months, new fat grafting improved the breast contour and
corrected the minor deformities. The patient progressed with good surgical acceptance,
and an ultrasound follow-up showed no changes resulting from the procedure (Figure 1).
Case 2. Elderly woman who had late breast reconstruction on the right with an ipsilateral
rectus abdominis muscle flap. She was dissatisfied with the result obtained due to
asymmetry between the breasts. The prosthesis was replaced by a 350 mL expander. After
six months, this expander was replaced by an anatomical silicone prosthesis of the
same volume and a 332 mL prosthesis with the same profile was placed in the left breast.
Fat grafting of 120 mL was used to correct small asymmetries and residual deformities.
Edema, pain and mild initial hyperemia disappeared after 15 to 30 days. The patient
was satisfied with the result obtained and presented no complications during outpatient
follow-up (Figure 2).
Case 3. Young patient who underwent immediate breast reconstruction with a 450 mL expander
prosthesis. After the end of adjuvant treatment with radiotherapy, the expander was
replaced by a 495 mL silicone prosthesis in the right breast, and a 250 mL was placed
in the left breast, with preoperative breast fat grafting for edge refinement and
creation of a new nipple-areolar complex (Figure 3).
CONCLUSION
Fat grafting is a noninvasive, safe, simple, and effective procedure. It has an excellent
indication in breast reconstruction for post-reconstruction refinements and secondary
contour defects. It is also used to improve tissue quality in irradiated breasts and
to replace the total volume of the breast, as seen in recent studies6,18,19.
Breast volume measurement after fat grafting is essential for long-term follow-up.
Most of the methods discussed in this review were presented in older publications,
including the anthropometric method, the thermoplastic models, and the Archimedes’
water displacement principle. These are outdated methods compared to the most modern
and reliable volume measurement techniques, such as MR and 3D body surface scanning20.
Although studies on fat grafting procedures in the last 15 years were successful,
no Level I or II data has yet justified the recommendation of a consensual protocol
for clinical practice2,5,8,9,11,12,14,18.
In the reported cases, fat grafting associated with breast expanders and prostheses
obtained satisfactory results for the patient and surgical team. It was used to increase
breast volume, improve the skin and support tissue structure, as well as refine and
correct minor imperfections after surgery.
COLLABORATIONS
|
CCO
|
Analysis and/or data interpretation, Conceptualization, Data Curation, Final manuscript
approval, Formal Analysis, Funding Acquisition, Methodology, Project Administration,
Realization of operations and/or trials, Resources, Validation, Writing - Original
Draft Preparation, Writing - Review & Editing
|
|
CCS
|
Conception and design study, Supervision
|
REFERENCES
1. Pereira Filho O, Ely JB. Lipoenxertia mamária seletiva. Arq Catarin Med. 2012;41(Supl
1).
2. Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy.
Plast Reconstr Surg. 2007;119(3):75-85.
3. Weichman KE, Broer PN, Tanna N, Wilson SC, Allan A, Levine JP, et al. The role of
autologous fat grafting in secondary microsurgical breast reconstruction. Ann Plast
Surg. 2013;71(1):24-30.
4. Costantini M, Cipriani A, Belli P, Bufi E, Fubelli R, Visconti G, et al. Radiological
findings in mammary autologous fat injections: a multi-technique evaluation. Clin
Radiol. 2013;68(1):27-33. DOI: https://doi.org/10.1016/j.crad.2012.05.009
5. Delay E, Garson S, Tousson G, Sinna R. Fat injection to the breast: technique, results,
and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29(5):360-76.
6. Gardani M, Bertozzi N, Grieco MP, Pesce M, Simonacci F, Santi PL, et al. Breast reconstruction
with anatomical implants: a review of indications and techniques based on current
literature. Ann Med Surg. 2017;21:96-104.
7. Strong AL, et al. The current state of fat grafting: a review of harvesting, processing,
and injection techniques. Plast Reconst Surg. 2015;136(4):897-912.
8. Illouz YG. Body contouring by lipolysis: a 5-year experience with over 3000 cases.
Plast Reconstr Surg. 1983;72:591-7.
9. Fournier PF. Fat grafting: my technique. Dermatol Surg. 2000;26(12):1117-28.
10. Illouz YG. Surgical remodeling of the silhouette by aspiration lipolysis or selective
lipectomy. Aesthet Plast Surg. 1985;9(1):7-21.
11. Carvajal J, Patiña JH. Mammographic findings after breast augmentation with autologous
fat injection. Aesthet Surg J. 2008;28(2):153-62.
12. Blumenschein AR, Freitas-Junior R, Tuffanin AT, Blumenschein DI. Lipoenxertia nas
mamas: procedimento consagrado ou experimental?. Rev Bras Cir Plást. 2012;27(4):616-22.
13. Har-Shai Y, Lindenbaum ES, Gamliel-Lazarovich A, Beach D, Hirshowitz B. An integrated
approach for increasing the survival of autologous fat grafts in the treatment of
contour defects. Plast Reconstr Surg. 1999;104(4):945-54.
14. Özkaya Ö, Egemen O, Barutça SA, Akan M. Long-term clinical outcomes of fat grafting
by low- pressure aspiration and slow centrifugation (Lopasce technique) for different
indications. J Plast Surg Hand Surg. 2013;47(5):394-8.
15. Pu LLQ, Coleman SR, Cui X, Ferguson Junior RE, Vasconez HC, et al. Autologous fat
grafts harvested and refined by the Coleman technique: a comparative study. Plast
Reconstr Surg. 2008;122(3):932-7.
16. Salgarello M, Visconti G, Rusciani A. Breast fat grafting with platelet-rich plasma:
a comparative clinical study and current state of the art. Plast Reconstr Surg. 2011;127(6):2176-85.
17. Herold C, Ueberreiter K, Busche MN, Vogt PM. Autologous fat transplantation: volumetric
tools for estimation of volume survival. A systematic review. Aesthet Plast Surg.
2013;37(2):380-7.
18. Kakudo N, Kushida S, Minakata T, Suzuki K, Kusomoto K. Platelet-rich plasma promotes
epithelialization and angiogenesis in a splitthickness skin graft donor site. Med
Mol Morphol. 2011;44(4):233-6.
19. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis
from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502-8.
20. Gentile P, Scioli MG, Orlandi A, Cervelli V. Breast reconstruction with enhanced stromal
vascular fraction fat grafting: what is the best method?. Plast Reconstr Surg Global
Open. 2015;3(6):e406.
1. Hospital Geral de Fortaleza, Fortaleza, CE, Brazil.
2. Instituto do Câncer do Ceará, Fortaleza, CE, Brazil.
Corresponding author: Carlos Cunha Oliveira Ávila Goulart, 900, Papicu, Fortaleza, CE, Brazil. Zip Code: 60175-295. E-mail: carloscunhaoliveira@hotmail.com
Article received: December 2, 2018.
Article accepted: October 20, 2019.
Conflicts of interest: none.