INTRODUCTION
Rhinoplasty is a surgery that, even in experienced hands, presents unpredictable results,
due to the anesthetic risks, possible functional and esthetic sequelae, and the necessary
recovery period. As a result, rhinomodelation with fillers has been gaining popularity
among both patients and medical professionals1,2.
The idea arose at the end of the 19th century by Dr. Robert Gersuny, who used paraffin
with the aim of increasing the nasal dorsum. Decades later, Robert Kotler and Jack
Startz introduced silicone injections, leading to a high rate of granulomas and ulcers.
In 1981, bovine collagen was introduced as the first facial filler approved for cosmetic
use3; however, with the need for a safer product, calcium hydroxyapatite (CaHA) was used
to shape some defects in the nose4,5. Subsequently, with the popularization and experience amassed by plastic surgeons
and dermatologists using hyaluronic acid (HA), this became the most commonly used
filler due to its reversibility with the use of hyaluronidase in the event of hypercorrection
or inadvertent vascular lesions and its lower durability when compared to CaHA6.
Some complications reported from the use of fillers (both HA and CaHA) include infection,
ischemia, and necrosis due to vasospasm, intra-arterial injection, or extrinsic vascular
compression in injections of large volumes at the tip or other areas with little tissue
distensibility, chronic pain, or formation of osteophytes by injection in the periosteum,
or ischemia in the dermis and epidermis from very superficial injections2.
OBJECTIVE
Our objective was to describe in detail an application technique of nasal fillers,
taking into account safety aspects, and to present a comparison between various characteristics
of CaHA and HA, expanding the vision of their properties to facilitate in choosing
the most suitable filler for each case.
METHODS
A retrospective analysis was performed of all patients who attended the clinic between
2009 and 2012 seeking improvement of their appearances through rhinomodelation and
who did not wish to undergo a rhinoplasty.
Patients with clinical diseases contraindicating general anesthesia; those with functional
breathing alterations; those under 20 and over 60 years; those with known allergies
to HA or CaHA; who previously underwent nose filling procedures, rhinoplasty surgery,
or fixation threads in the nose, and those with changes in the nose with the indication
of rhinoplasty surgery were excluded.
All patients signed an informed consent form, and the rhinomodelation product (HA
or CaHA) was chosen according to the information provided during the consultation.
A plastic surgeon performed the application of the product.
All patients were reassessed the day following the procedure and after 14 days, at
which point the product was reapplied in cases that the surgeon or patient considered
the initial nasal deformity to require additional correction.
Edema of the nasal tip was evaluated on the day after the procedure by the subjective
opinion of the professional who performed the application, taking into account the
intensity of skin erythema, the hardness of the treated area upon palpation, and the
increase in the tip volume. The edema was evaluated on a scale from no edema to mild,
moderate, or intense edema.
Pain was evaluated on the day following the procedure by the patient by slightly pressing
the nasal tip with the finger pad of the index finger of the dominant hand and measuring
the pain on a scale of 1 to 5 (1=no pain, 2 = mild pain, 3=moderate pain, 4=intense
pain and 5=unbearable pain).
The degree of satisfaction with the results was evaluated after 2 weeks by the patient
assessing the pre- and post-procedure photographs before any reapplication, and it
was measured on a scale from 1 to 5 (1=very satisfied, 2=satisfied, 3=slightly satisfied,
4=dissatisfied and 5=totally dissatisfied, would not recommend and would not do it
again).
Technique
After adequate antisepsis of the face with aqueous chlorhexidine, a topical anesthetic
containing lidocaine 23% + tetracaine 7% gel was applied for 10 minutes, followed
by the application of the dermal filler (CaHA or HA).
Application in the nasal spine: the syringe and needle were placed in direct orientation
to the nasal spine joining the nasolabial angle (Figure 1).
Figure 1 - Application in the nasal spine.
Figure 1 - Application in the nasal spine.
Recommendations: This was used for cases with closed nasolabial angles (less than
95-100 degrees in women and 93-98 degrees in men7). When feeling the bone with the tip of the needle, retreat 1-2 mm; aspirate to avoid
an intra-arterial application and then delicately place the product, observing the
opening of the nasolabial angle. This area does not need much digital modeling, but
it requires compression to avoid ecchymosis.
Application in the columella: ingress from the base towards the nasal tip in the midline.
The product is placed between the medial crus of each alar cartilage and the caudal
septum (Figure 2).
Figure 2 - Application in the columella.
Figure 2 - Application in the columella.
Recommendations: This was used for cases with an easily depressible tip and a weak
columella. The needle is inserted at an angle of 45 degrees to facilitate its application.
The syringe is aspirated and the application of the product is delicately done in
a retrograde direction, repeating the application as many times as necessary to achieve
the result. The volume is large enough to provide support to the columella but not
to leave it large and irregular. The objective is analogous to a columellar strut
to sustain the tip. Modelling and digital compression for one minute are necessary,
raising the nasal tip cranially.
Application in the tip: The ingress is straight up to the interdomal space (Figure 3).
Figure 3 - Application in the tip.
Figure 3 - Application in the tip.
Recommendations: This is used for cases that are ill-defined, with a round tip and
without projection. A straight needle is used to aspirate and delicately apply the
material with low pressure in the retrograde direction. The color of the tissue is
observed throughout the procedure, paying attention to any sign of ischemia (mottling,
paleness, purplish coloration, reduction of temperature, or excessive redness). Immediate
modeling and digital compression for one minute are necessary, providing shape and
finesse to the tip, seeking to shape the product in such a way as to produce a triangular
or diamond shape to the nasal tip.
Application in the dorsum: the ingress is straight up to the supraperiosteal space
of the nasal bone in the cranial direction (Figure 4).
Figure 4 - Application in the dorsum.
Figure 4 - Application in the dorsum.
Recommendations: This is used for cases with irregularities or depressions on the
dorsum. Needle ingress is held at an angle of 30 degrees to aspirate and delicately
apply the material, spreading the product symmetrically with the help of the other
hand. Lifting the skin and pinching it helps to find the correct plane. Superficial
application is avoided in order to not provoke the Tyndall effect in the skin, and
scraping the needle in the periosteum is avoided to prevent pain or periosteal reaction.
Immediate digital modeling and delicate compression for one minute are necessary.
After the application, skin-colored paper tape is placed over the nose in the shape
of a usual rhinoplasty until the following day.
RESULTS
Forty-two patients seeking nasal filling and who met the selection criteria were evaluated;
2 patients were excluded (they did not return for the 14 day evaluation). One patient
had signs of hypoperfusion when hyaluronic acid was applied in the nasal tip (skin
pallor), so hyaluronidase was used, providing a satisfactory evaluation the next day.
This was considered a complication, but it was not included in the analysis due to
variations that it would cause in the evaluation of the results. A total of 39 patients
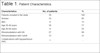
were included in this study. Patient characteristics are summarized in Table 1.
Table 1 - Patient Characteristics.
| Characteristics |
No. of patients |
% |
| Patients included in the study. |
39 |
100 |
| Women |
33 |
85 |
| Men |
6 |
15 |
| Age 20-40 years |
30 |
77 |
| Age 40-60 years |
9 |
23 |
| Rhinomodelation with HA |
27 |
69 |
| Rhinomodelation with CaHA |
12 |
31 |
| Complications with hypoperfusion (HA) |
1 |
- |
Table 1 - Patient Characteristics.
The results evaluated the following day and at 14 days are summarized in Table 2 and Figures 5-12.
Table 2 - Results.
| Characteristics evaluated |
HA (N: 27) |
CaHA (N: 12) |
| Edema |
| Without edema |
0 |
0 |
| Mild edema |
14 (52%) |
4 (33%) |
| Moderate edema |
13 (48) |
8 (67%) |
| Moderate edema |
0 |
0 |
| Pain |
| Without pain |
0 |
0 |
| Mild pain |
20 (74%) |
4 (33%) |
| Moderate pain |
7 (26%) |
6 (50%) |
| Intense pain |
0 |
2 (17%) |
| Unbearable pain |
0 |
0 |
| Degree of satisfaction (at 14 days) |
| Very satisfied |
4 (15%) |
10 (84%) |
| Satisfied |
20 (74%) |
0 |
| Not very satisfied |
3 (11%) |
2 (16%) |
| Dissatisfied |
0 |
0 |
| Totally dissatisfied, would not recommend, and would not do it again |
0 |
0 |
| Reapplication of the product |
24 (89%) |
2 (17%) |
Figure 5 - Frontal, oblique, and side aspects. Top line: Before. Bottom line: After. Before and
after 2 weeks of rhinomodelation with HA in the nasal spine, columella and tip of
the dorsum.
Figure 5 - Frontal, oblique, and side aspects. Top line: Before. Bottom line: After. Before and
after 2 weeks of rhinomodelation with HA in the nasal spine, columella and tip of
the dorsum.
Figure 6 - Frontal, oblique, and side aspects. Top line: Before. Bottom line: After HA. Before
and after 2 weeks of rhinomodelation with HA in the nasal spine, columella, and tip
of the dorsum.
Figure 6 - Frontal, oblique, and side aspects. Top line: Before. Bottom line: After HA. Before
and after 2 weeks of rhinomodelation with HA in the nasal spine, columella, and tip
of the dorsum.
Figure 7 - Pre-procedure and immediate result of rhinomodelation with CaHa in the nasal spine,
columella, nasal tip and dorsum.
Figure 7 - Pre-procedure and immediate result of rhinomodelation with CaHa in the nasal spine,
columella, nasal tip and dorsum.
Figure 8 - Pre-procedure and immediate result of rhinomodelation with CaHa in the nasal spine,
columella, nasal tip and dorsum.
Figure 8 - Pre-procedure and immediate result of rhinomodelation with CaHa in the nasal spine,
columella, nasal tip and dorsum.
Figure 9 - Pre-procedure and immediate result of rhinomodelation with CaHa in the nasal spine,
columella, nasal tip, and dorsum
Figure 9 - Pre-procedure and immediate result of rhinomodelation with CaHa in the nasal spine,
columella, nasal tip, and dorsum
Figure 10 - Pre-procedure and at 14 days after rhinomodelation with HA in the columella, nasal
tip, and dorsum.
Figure 10 - Pre-procedure and at 14 days after rhinomodelation with HA in the columella, nasal
tip, and dorsum.
Figure 11 - Pre-procedure and immediate result of rhinomodelation with HA in the nasal spine,
columella, nasal tip, and dorsum.
Figure 11 - Pre-procedure and immediate result of rhinomodelation with HA in the nasal spine,
columella, nasal tip, and dorsum.
Figure 12 - Pre-procedure and immediate result of rhinomodelation with CaHa in the nasal spine,
columella, nasal tip, and dorsum.
Figure 12 - Pre-procedure and immediate result of rhinomodelation with CaHa in the nasal spine,
columella, nasal tip, and dorsum.
Patients who were not very satisfied with the HA reported little change in the results.
Patients who were not very satisfied with the CaHA reported discomfort due to edema
and pain in the nose.
DISCUSSION
Discrete volumetric variations in the frontonasal angle, nasal dorsum, and nasolabial
angle lead to significant differences in our perception of nasal esthetics2.
With respect to rhinomodelation, several factors must be considered before choosing
the product. Two of the most important characteristics of these products to be considered
are elasticity (ability to resist deformation) and viscosity (ability to resist applied
force preventing propagation) of the product8. Therefore, a filler with high elasticity and viscosity provides greater support
while using a smaller volume9.
On the tissue application plane, we recommend the supraperiosteal use of CaHA and
HA in the subcutaneous tissue or deep dermis of all other nasal areas that can be
corrected, such as the dorsum, tip, columella, and nasolabial angle10. Regarding the durability of the product, the literature shows a lifespan of 6-30
months in patients treated with CaHA11. As for the use of other products as a complement, we recommend the use of botulinum
toxin type A (12U) in all cases that present hyperactivity of the depressor muscle
of the septum12.
Although most researchers do not staunchly prefer one or the other, HA has become
the most commonly used filler due to the safety offered by hyaluronidase, an enzyme
that allows a certain degree of reversibility to the action of HA13. In cases with signs of poor perfusion, the application of HA should be stopped,
the area kneaded, and hyaluronidase injected (10 units per each 0.1 ml of HA injected).
The use of antiplatelets, peripheral vasodilators, or hyperbaric oxygen can also be
useful6.
In our study, we found that HA led to a higher incidence of edema and mild pain, while
CaHA led to a higher percentage of edema and moderate and intense pain on the day
following the procedure. The necessity of reapplication in 2 weeks was less frequent
with the use of the CaHA, which is in agreement with the literature8,14-15. Both products presented a high degree of satisfaction with the esthetic results.
Some limitations of this study include the limited number of patients, the subjective
evaluation by a single professional of the edema, the non-evaluation of the durability
of each product, the short follow-up period (2 weeks), and the fact that possible
differences between different brands of both products were not assessed.
CONCLUSIONS
Rhinomodelation with resorbable fillers is a simple procedure with acceptable esthetic
results in selected cases, and a deep anatomical knowledge is necessary to decrease
the risk of complications.
COLLABORATIONS
|
RMR
|
Analysis and/or data interpretation, Data Curation, Final manuscript approval, Investigation,
Methodology, Writing - Review & Editing
|
|
HEB
|
Writing - Review & Editing
|
|
PSP
|
Final manuscript approval, Methodology, Writing - Original Draft Preparation, Writing
- Review & Editing
|
|
ES
|
Analysis and/or data interpretation, Conception and design study, Data Curation, Final
manuscript approval, Supervision
|
REFERENCES
1. Lin G, Lawson W. Complications using grafts and implants in rhinoplasty. Operative
Techniques in Otolaryngology-Head and Neck Surgery. 2007;18(4):315-323.
2. Bray D, Hopkins C, Roberts DN. Injection rhinoplasty: Non-surgical nasal augmentation
and correction of post-rhinoplasty contour asymmetries with hyaluronic acid: How we
do it. Clin Otolaryngol. 2010 Jun;35(3):227-230.
3. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg.
2009 May;25(2):67-72.
4. Rivkin A, Soliemanzadeh P. Nonsurgical injection rhinoplasty with calcium hydroxyapatite
in a carrier gel Radiesse: A 4-year retrospective clinical review. Cosmet Dermatology.
2009;22:619-624.
5. Stupak H, Moulthrop TH, Wheatley P, Tauman AV, Johnson Junior CM. Calcium hydroxylapatite
gel (Radiesse) injection for the correction of postrhinoplasty contour deficiencies
and asymmetries. Arch Facial Plast Surg. 2007 Mar/Apr;9(2):130-136.
6. Kurkjian TJ, Ahmad J, Rohrich RJ. Soft-tissue fillers in rhinoplasty. Plast Reconstr
Surg. 2014 Feb;133(2):121e-6e.
7. Armijo BS, Brown M, Guyuron B. Defining the ideal nasolabial angle. Plast Reconstr
Surg. 2012;129(3):759-64.
8. Jasin ME. Nonsurgical rhinoplasty using dermal fillers. Facial Plast Surg Clin North
Am. 2013 May;21(2):241-252.
9. Sundaram H, Voigts B, Beer K, Meland M. Comparison of the rheological properties of
viscosity and elasticity in two categories of soft tissue fillers: Calcium hydroxyapatite
and hyaluronic acid. Dermatol Surg. 2010 Nov;36(Suppl 3):1859-1865.
10. Helmy Y. Non-surgical rhinoplasty using fillers, Botox and thread remodeling: Retro
analysis of 332 cases. J Cosmet Laser Ther. 2018 Oct;20(5):293-300.
11. Schuster B. Injection rhinoplasty with hyaluronic acid and calcium hydroxyapatite:
A retrospective survey investigating outcome and complication rates. Facial Plast
Surg. 2015 Jun;31(3):301-7.
12. Redaelli A. Medical rhinoplasty with hyaluronic acid and botulinum toxin A: A very
simple and quite effective technique. J Cosmet Dermatol. 2008 Sep;7(3):210-20.
13. Kim DW, Yoon ES, Ji YH, Park SH, Lee BI, Dhong ES. Vascular complications of hyaluronic
acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet
Surg. 2011 Dec;64(12):1590-5.
14. Becker H. Nasal augmentation with calcium hydroxyapatite in a carrier-based gel. Plast
Reconstr Surg. 2008 Jun;121(6):2142-7.
15. Rokhsar C, Ciocon DH. Nonsurgical rhinoplasty: An evaluation of injectable calcium
hydroxylapatite filler for nasal contouring. Dermatol Surg. 2008 Jul;34(7):944-6.
1. Be-You Medical Spa, Cirurgia Plástica, Lima, Lima, Peru.
2. Hospital São Lucas da Pontifícia Universidade Católica do Rio Grande do Sul, Porto
Alegre, RS, Brazil.
3. Hospital Santa Casa da Misericórdia do Rio de Janeiro, Rio de Janeiro, RJ, Brazil.
Corresponding author: Renato Matta Ramos Be-You Medical Spa, Calle Libertadores, 125, San Isidro, Lima, Perú. Zip code: 15073.
E-mail: renatomatta82@hotmail.com
Article received: October 14, 2018.
Article accepted: April 21, 2019.
Conflicts of interest: none.