INTRODUCTION
Breast augmentation with a silicone implant is one of the most common plastic surgery
procedures in Brazil and worldwide1-3. Considering that some of these patients may need some type of secondary intervention4-7, ranging from minor scar-repairing procedures to complex surgeries for complete breast
reconstruction, it is important for plastic surgeons to be prepared to meet patient
expectations and deal with possible difficulties.
Over the last decades, the rate of reoperations for breast augmentation has remained
unchanged at about 20% after 3 years regardless of the type of implant8.
Therefore, it is important to know different secondary surgical methods in order to
provide solutions for complex cases, inadequate results, and patients dissatisfied
with primary surgery results.
OBJECTIVE
The objective of this study was to evaluate patients with previous silicone implantations
undergoing secondary mammaplasty, presenting an alternative approach with en block
resection of breast tissue, fibrous capsule, and silicone implant, followed by implant
repositioning in the partial retropectoral pocket.
Methods
This was a cross-sectional retrospective study to analyze medical records and photographic
documentation of patients who had undergone a primary breast implant surgery at least
6 months before the secondary breast surgery in the period from January 2013 to March
2017. All patients were operated by the author in his private clinic.
The analysis followed the principles of the Declaration of Helsinki (2000) and resolution
466/2012 of the National Health Council regarding ethical and legal aspects of research
involving human beings in Brazil.
This study included 24 cases of breast surgery with en block resection and implant
replacement and repositioning in the partial retropectoral pocket during the abovementioned
period.
The patients were operated by the same professional, regardless of where the primary
intervention was conducted. After an initial consultation with general evaluation
and surgical planning, the patients underwent clinical and cardiological evaluation
and were considered fit for the procedure.
The patients underwent laboratory screening, chest radiographic examinations, and
breast imaging exams. According to age and need for diagnostic clarification, some
patients underwent breast ultrasound, mammography, and/or nuclear magnetic resonance
imaging. Patients had a consultation preferably one day before surgery for photographic
documentation and clarification of any questions.
The surgery indication was based on the presence of at least one of the following
criteria: patient’s motivation to improve breast esthetics; and changes on physical
examination and/or imaging exams that justified mammaplasty with implant replacement.
Therefore, the objectives of the surgery were defined considering, above all, the
patient’s expectations regarding breast size.
Progress and results were evaluated by comparative physical examination and using
photographic records 60 days and 6 months after the procedure at regular postoperative
consultations and considering the patients’ validation of results. Ultrasound examination
was requested for all cases 6 months after the procedure to verify proper placement
of the breast implants.
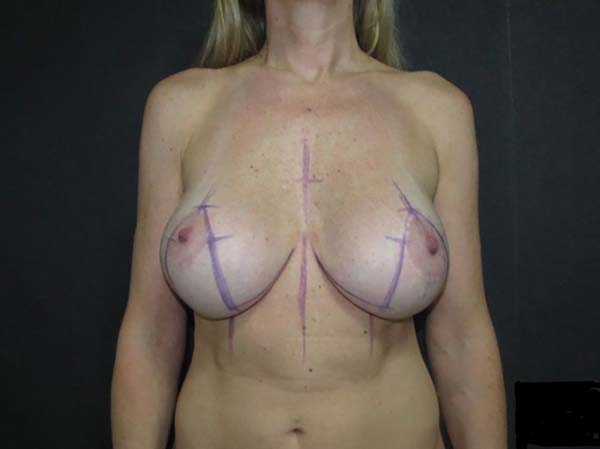
Preoperative marking
After marking the midline throughout the chest, breast markings began at point A,
bilaterally. Point A corresponded to the new position of the nipple-areola complex
(NAC), and so it was positioned on the mammary midline above the anterior projection
of the mammary fold9-12. Points A were marked strictly equidistant to the thoracic midline (TML) and to the
sternal notch to identify and correct NAC asymmetries.
Vertical lines were delimited by skin traction (medially and laterally) in relation
to a point on the mammary fold (called point X), positioned at a distance 1 to 2 cm
shorter than the distance from point A to the TML. After the vertical markings were
defined, the points corresponding to the height of the lower border of the new areola
position (called points B and C) and the future point of the junction of the vertical
incisions in the mammary fold (called points D and E) were marked.
The distances of points B and C and D and E from point A were between 3.5 and 4 cm
and 10 and 11 cm, respectively. This marking was based on overall breast characteristics
(skin, breast tissue density, base diameter, and need for projection) and size of
the implant to be used.
Next, horizontal resection lines were delimited by lines marked between points D and
E and the medial and lateral extremities of the mammary fold line. During these markings,
skin traction was very carefully applied so that points D and E were more distant
than point X from the medial and lateral borders of the inframammary fold scar, in
order to prevent over-resection and excessive tension in vertical sutures, especially
with the use of larger implants.
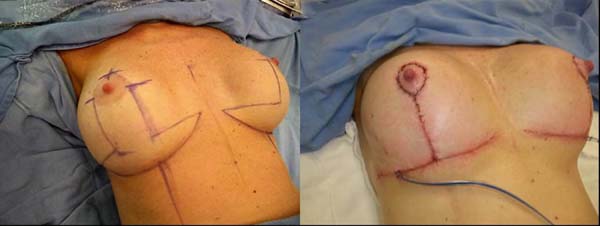
Subsequently, all markings were photographed (Figure 1) and the positions of the future scars were shown to the patient.
Figure 1 - Preoperative marking.
Figure 1 - Preoperative marking.
The patients underwent general anesthesia or epidural block associated with intravenous
sedation in a hospital environment, respecting the anesthetic criteria and the joint
decision of the anesthetist and the patient. Analgesic, anti-inflammatory, and antibiotic
medications were used during and after surgery.
Operative technique
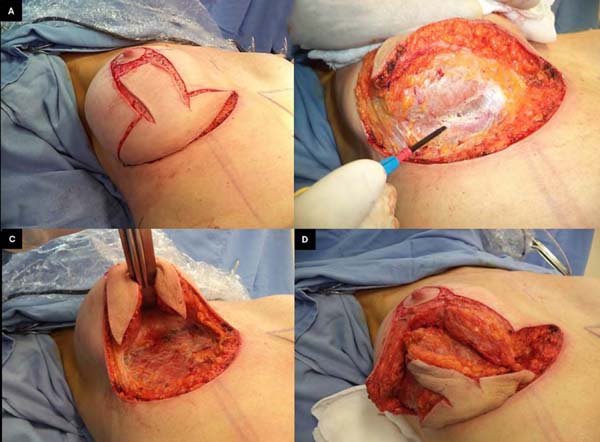
The patient was placed in the supine position with approximately 30° of elevation;
skin incisions on the previous marking lines and de-epidermization of the periareolar
skin were then performed (Figure 2A) for subsequent superior rotation of the NAC with the superior or superomedial pedicle13, according to the previously defined point A position or the need to adapt to breast
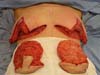
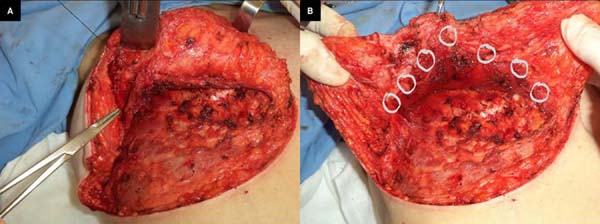
tissue resections and size of the new implant. En bloc resection included excess skin
and breast tissue at the lower breast pole, capsule, and implant (Figures 2B, 2C, and 2D). Microdissection needles (Colorado Type, Black & Black Surgical, Inc.) were used
for electrocautery detachment to facilitate hemostasis and the perfect separation
of the capsule and surrounding tissues.
Figure 2 - En bloc resection of the skin, breast tissue, fibrous capsule, and implant.
Figure 2 - En bloc resection of the skin, breast tissue, fibrous capsule, and implant.
After complete resection of the mammary tissue, capsule, and implant, no scar tissue
or residual capsule was left. The detached area was then exhaustively washed with
0.9% saline solution and protected with a wet compresses for subsequent hemostatic
testing when necessary.
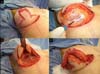
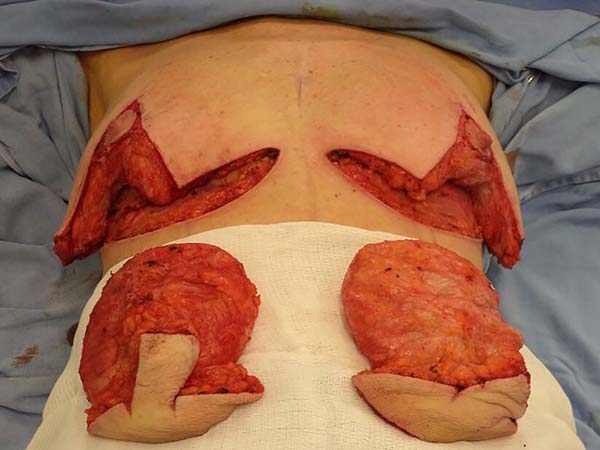
The same procedure was performed on the contralateral breast, followed by bilateral
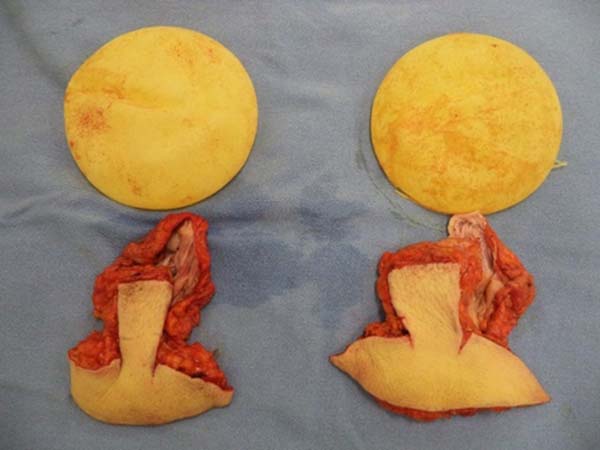
total en bloc resection of the structures (Figure 3). The capsule was opened outside the surgical field for analysis of integrity, deformities,
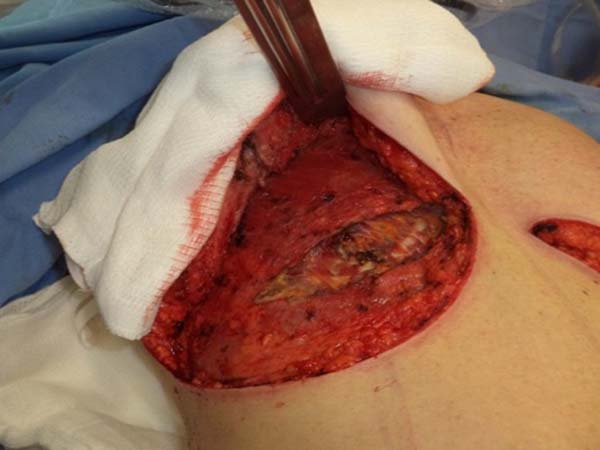
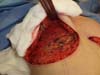
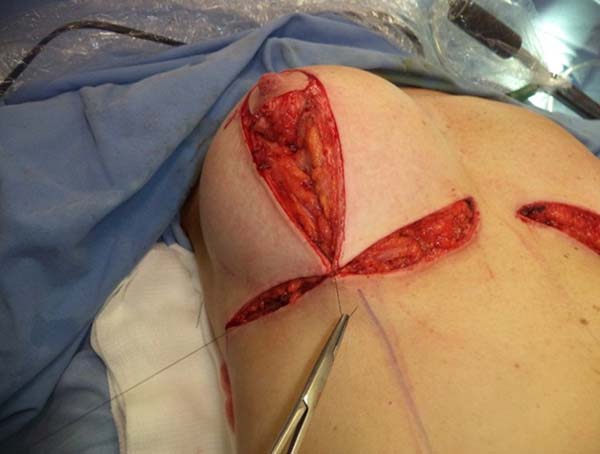
type, and size of the patient’s implant (Figure 4). After delimitation and symmetrization of the detached areas in both breasts, bilateral
retromuscular pockets were made starting with the incision of the pectoral muscle
in the nearest portion of the mammary fold3,7 (Figure 5).
Figure 3 - Bilateral resection.
Figure 3 - Bilateral resection.
Figure 4 - Separation of implants and breast tissue.
Figure 4 - Separation of implants and breast tissue.
Figure 5 - Incision in the pectoralis major muscle to prepare a retromuscular pocket.
Figure 5 - Incision in the pectoralis major muscle to prepare a retromuscular pocket.
After detachment of the pectoralis major muscle up to the medial insertion limit -
as necessary to accommodate the new implant and to avoid superior displacement due
to pressure resulting from muscular action - hemostasis was rigorously tested in the
anterior and posterior regions of the muscle, bilaterally.
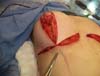
The pectoral muscle was sutured to the breast tissue to facilitate implant accommodation
in the retromuscular pocket, avoiding migration of the implant to the anterior part
of the muscle and providing muscle flap stability to the breast tissue. Suturing was
started with a stitch on the mammary midline corresponding to the point of support
at the level of the 4th or 5th intercostal space and NAC projection (Figure 6A). After that, two to four stitches were made on each side along the lower border
of the pectoralis major muscle using Mononylon® 3.0 and inverted knot sutures so that the thread was not in direct contact with the
silicone implant (Figure 6B).
Figure 6 - Suture of the pectoral muscle and breast tissue.
Figure 6 - Suture of the pectoral muscle and breast tissue.
After the implant (textured, round, high or super high profile, Eurosilicone, Mentor
Corporation, or Natrelle brands) was accommodated in a partial retromuscular pocket,
mammary suture was started at the junction of the pillars at the X-point projection,
respecting anatomical planes and perfect accommodation of mammary, subdermal, and
dermal tissue (Figure 7).
Figure 7 - Plane suture restructuring of the breast on the implant.
Figure 7 - Plane suture restructuring of the breast on the implant.
Closed suction drains were used for 1 to 5 days, depending on the volume and appearance
of the drained fluid. The drains were placed in the submuscular pocket with some holes
in the inferior lateral portion of the breast (Figure 8B). Right after surgery, it was possible to see adequate mammary tissue coaptation
with the implant and greater breast support compared to those preoperatively (Figures 8A and 8B).
Figure 8 - Immediate result.
Figure 8 - Immediate result.
All patients received verbal and written instructions on specific postoperative care
in order to avoid implant displacement, especially in the first 60 postoperative days.
RESULTS
The mean age of the operated patients was 50 years (minimum 24 and maximum 73 years)
and the mean time since the first implant surgery was 10.1 years (minimum 1 year and
maximum 25 years).
This study included 24 secondary mammaplasty procedures involving mammary implant
replacement and repositioning in the partial retropectoral pocket during the study
period. All cases presented breast changes on physical examination, including breast
ptosis (moderate to severe), capsular contracture, improper implant positioning, and
breast asymmetries.
Dissatisfaction with the results of the primary surgery was reported in 10 (41.6%)
cases, and most of these patients had undergone breast augmentation mammaplasty less
than 10 years before. The other 14 (58.4%) patients reported being satisfied with
primary surgery results; however, the changes that appeared over time motivated them
to undergo a new surgery.
Implant-related changes in image examinations (mammography, ultrasound, and/or nuclear
magnetic resonance) were described in 7 (29.1%) cases. Capsular contracture (Baker
Classification II14 or above) was identified during physical examination and in imaging exams in 7 (29.1%)
cases, and 2 (8.3%) cases showed evidence of intracapsular implant rupture. Six (25%)
primary breast augmentation procedures were performed by the author and 18 (75%) by
other professionals.
Of the cases in which the patient’s clinical history was not available, the implant
volume was accurately reported or some type of implant documentation was maintained
in only 4 (22.2%). Thus, implants with volumes different than those reported by patients
were found in 14 cases, corresponding to 77.7% of surgeries. Implant volume could
not be identified in one case because the patient had no relevant information on volume
and it was a ruptured smooth implant. It was the oldest implant (25 years) analyzed.
During surgery, textured implants were identified in 16 (66.6%) patients, polyurethane-coated
implants in 7 (29.1%), and smooth implants in 1 (4.1%).
The mean volume of the implants replaced during surgery was 233 cc (minimum 135 cc
and maximum 300 cc) on the right side and 235 cc (minimum 135 cc and maximum 375 cc)
on the left side. The mean volume of the implants repositioned during surgery was
341 cc (minimum 200 cc and maximum 450 cc) on the right side and 341 cc (minimum 220
cc and maximum 450 cc) on the left side. The mean volume of the implants used was
the same for both sides, although different sized implants were used to compensate
for asymmetries that could not be corrected only by resecting excess skin and breast
tissue. Implant volume tends to be larger in secondary surgeries to compensate for
glandular atrophy and provide greater breast tissue support, especially when placed
in the retromuscular plane.
Table 1 shows information on patients’ complaints, medical evaluation, types of implant coating,
and volume of implants replaced and repositioned in this study.
Table 1 - Clinical information and breast implant details of the cases analyzed in the study.
| Patient |
Age |
Time |
Contracture |
Rupture |
Asymmetry |
Ptosis |
Image |
Dissatisfaction |
Others |
Type
of implant
|
Right Pre |
Right Post |
Left Pre |
Left Post |
| CF |
45 |
5 |
|
|
X |
|
|
X |
X |
Textured |
260 |
350 |
260 |
350 |
| SPP |
54 |
8 |
|
|
|
X |
|
|
|
Textured |
280 |
325 |
280 |
325 |
| TPB |
62 |
25 |
X |
X |
X |
X |
X |
|
X |
Smooth |
|
280 |
|
280 |
| GP |
73 |
1 |
|
|
|
X |
|
X |
X |
Textured |
220 |
200 |
220 |
220 |
| MDV |
52 |
9 |
|
|
|
X |
|
|
|
Textured |
240 |
325 |
240 |
325 |
| GDS |
61 |
17 |
X |
X |
X |
|
X |
|
X |
Polyurethane |
135 |
400 |
135 |
400 |
| IK |
41 |
9 |
|
|
|
X |
|
|
|
Textured |
300 |
300 |
300 |
300 |
| AS |
54 |
10 |
|
|
|
X |
X |
X |
X |
Polyurethane |
260 |
325 |
260 |
325 |
| FR |
38 |
10 |
X |
|
|
|
|
|
X |
Textured |
235 |
375 |
235 |
375 |
| MG |
54 |
20 |
|
|
X |
|
|
X |
X |
Textured |
260 |
375 |
260 |
375 |
| VP |
47 |
8 |
|
|
|
X |
|
|
|
Textured |
220 |
325 |
220 |
325 |
| RS |
47 |
12 |
|
|
|
X |
|
X |
X |
Textured |
175 |
375 |
175 |
375 |
| IR |
60 |
2 |
X |
|
|
|
X |
|
X |
Polyurethane |
240 |
400 |
240 |
400 |
| MFR |
24 |
4 |
|
|
X |
|
|
X |
X |
Textured |
285 |
350 |
285 |
375 |
| LRL |
57 |
12 |
|
|
|
X |
|
|
|
Textured |
230 |
350 |
230 |
350 |
| EO |
39 |
4 |
|
|
X |
|
|
X |
X |
Textured |
250 |
350 |
250 |
350 |
| AA |
46 |
15 |
X |
|
|
X |
X |
|
X |
Polyurethane |
140 |
450 |
140 |
450 |
| JM |
33 |
3 |
|
|
X |
|
|
X |
X |
Textured |
260 |
350 |
260 |
350 |
| BV |
58 |
13 |
|
|
|
X |
|
|
|
Textured |
280 |
350 |
280 |
350 |
| CF |
70 |
17 |
X |
|
X |
|
X |
|
X |
Polyurethane |
190 |
300 |
190 |
300 |
| EA |
41 |
13 |
|
|
|
X |
|
|
X |
Polyurethane |
175 |
375 |
175 |
375 |
| IG |
51 |
10 |
X |
|
|
|
X |
X |
X |
Polyurethane |
155 |
240 |
155 |
240 |
| RO |
49 |
6 |
|
|
|
X |
|
|
X |
Textured |
300 |
310 |
375 |
265 |
| EC |
44 |
10 |
|
|
X |
X |
|
X |
X |
Textured |
285 |
420 |
255 |
420 |
Table 1 - Clinical information and breast implant details of the cases analyzed in the study.
There was no implant displacement, retroglandular migration, or breast asymmetry requiring
corrective surgery at the evaluation 60 days after the surgery, using physical examination,
photographic records, surgeon’s technical validation, and patients’ approval as parameters.
The patients had no complaints of residual excess skin at the time of this evaluation.
Mild to moderate mammary ptosis was reported in 3 (12.5%) cases, mainly related to
weight loss during the period, previous presence of stretch marks, and sagging body
skin. Additional skin resection was scheduled, without any need for direct intervention
on breast implants.
The patients underwent breast ultrasound examinations 6 months after the surgery,
which showed no implant rupture, contour irregularities, residual seroma, or other
changes related to the surgery analyzed in this study.
Surgical complications included 4 cases of unilateral suture dehiscence in the inframammary
fold, representing 8.3% of the 48 operated breasts. Partial unilateral areola necrosis
occurred in one patient, corresponding to 2.8% of the operated breasts. This specific
patient had undergone the primary procedure and two breast repair surgeries. All complications
were resolved under local anesthesia. Complete suture dehiscence, hematoma, infection,
or other major complications were not reported in the patients undergoing the procedure
described in this study.
DISCUSSion
Given the significant number of women undergoing breast augmentation or breast repositioning
surgeries with silicone implants in recent years, surgeons should find practical and
objective secondary mammaplasty solutions, providing satisfactory results to patients
and avoiding excessively long procedures or high blood loss.
In 2001, Melega et al.15 described the surgical approach for en bloc resection in cases of capsular contracture
correction through an incision on the previous scar, dissecting the fibrous capsule
with blunt scissors. This procedure was called “capsulectomy without capsulotomy”.
En bloc resection of the skin, mammary gland, fibrous capsule, and implant has multiple
benefits during a highly complex surgery such as mammaplasty associated with implant
replacement.
Some of the benefits are as follows:
a) Practical skin incisions: skin incision becomes practical with the support provided
by the implant and its fibrous capsule, often hardened by capsular contracture, allowing
precise incisions even on thin skin or with stretch marks;
b) Detachment plane control: implant stability prevents the capsule from bending during
traction, facilitating detachment of the medial, lateral, and posterior portions and
virtually eliminating the risk of capsule residues;
c) Hemostasis control: it is easier to visualize and cauterize blood vessels during
resection when the contour of the fibrous capsule is detached, keeping the operative
field clean and resulting in minimal blood loss;
d) Avoiding silicone and/or intracapsular secretion extravasation into the mammary
tissue: the risk of contamination in implant replacement surgeries is significantly
reduced without opening the capsule for implant removal. Even if the capsule is opened,
it is possible to easily aspirate the intracapsular liquid content, avoiding contact
of this content with adjacent tissues;
e) Objective mammary tissue resection and implant removal: reduced surgical time in
the initial phase of the procedure provides safer mammary restructuring, reducing
complications related to long-term surgery.
Different techniques to approach the fibrous capsule have been proposed in previous
studies15-20, many of them preserving the whole capsule or part of it, with favorable results.
However, the maintenance of fibrous tissue in contact with the implant, probably incorporating
silicone gel extravasation residues, can have disastrous consequences, especially
the occurrence of potential bacterial contamination and local infection, which would
result in implant removal15,21-24.
A broad review of the literature on capsular contracture management shows that both
capsulotomy and capsulectomy can be effective, suggesting total capsulectomy in cases
of retroglandular implant contracture25.
In 2006, Spear20 described a technique for capsular contracture correction with total or partial capsulectomy
and implant repositioning to the retropectoral plane using marionette half-mattress
sutures to obliterate the subglandular space, obtaining satisfactory results with
low risk of capsular contracture.
The complexity of the surgery and the instability of the structures mobilized in the
presented procedure evidently require preventive measures to avoid implant detachment
and migration to the retroglandular space, which would be disastrous, especially if
unilateral. Thus, we used resistant and non-absorbable thread for direct suture with
multiple stitches to achieve perfect adherence of the lower border of the pectoral
muscle to the mammary tissue.
Although implants are not completely covered by the pectoral muscle, covering the
superomedial portion of the implant provides a natural result. In addition, a careful
detachment of the lower part of the pectoral muscle and the maintenance of medial
and lateral insertions allow implant accommodation, providing greater stability and
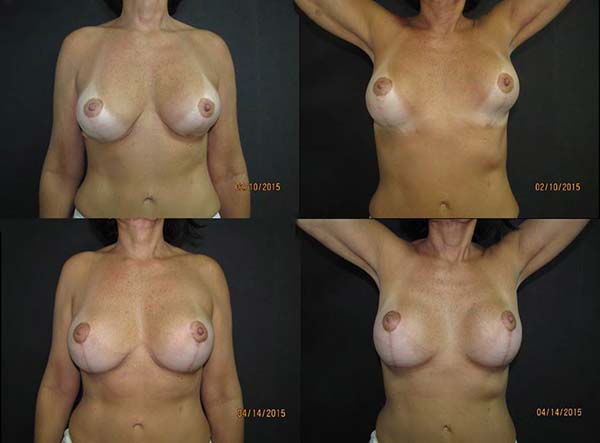
reduced risks of postoperative displacement3,19,26 (Figures 9A-9D).
Figure 9 - Results report.
Figure 9 - Results report.
In the studied cases, the use of polyurethane-coated implants and their complete adherence
to the fibrous capsule resulted in a more practical resection even with varying degrees
of capsular contracture. Fibrous capsules of textured implants were thinner, and implant
instability due to the presence of residual seroma or pockets bigger than necessary
resulted in more difficult resection.
The complication rate corroborated the results of previous studies, even considering
the complexity of the procedure and the fact that these are secondary breast surgeries2,4,5,15,19. No interventions were required for surgical repair of residual breast ptosis, implant
displacement, or breast asymmetries during the postoperative follow-up of the studied
cases. Moreover, none of the cases presented capsular contracture until this manuscript
was prepared. The results of this study show implant stability and low long-term capsular
contracture index, even considering the relatively short follow-up period.
Proper planning and implant positioning are essential in breast augmentation surgeries.
Therefore, it is important to know different secondary or reparative surgery methods
in order to provide solutions for complex cases, inadequate results, and patients
dissatisfied with primary surgery results.
CONCLUSION
En bloc resection and implant repositioning in the partial retropectoral pocket with
sutures attaching the pectoralis major muscle to breast tissue is an alternative to
improve secondary breast surgery, providing favorable results in cases of excessive
mobility or capsular contracture in implants initially placed in the retroglandular
position.
COLLABORATIONS
|
VJC
|
Analysis and/or data interpretation, Conception and design study, Data Curation, Final
manuscript approval, Methodology, Writing - Review & Editing
|
REFERENCES
1. Spear SL, Bulan EJ, Venturi ML. Breast augmentation. Plast Reconstr Surg. 2004 Oct;114(5):73E-81E.
PMID: 15457008
2. Pitanguy I, Amorim NFG, Ferreira AV, Berger R, Análise das trocas de implantes mamários
nos últimos cinco anos na Clínica Ivo Pitanguy. Rev Bras Cir Plást. 2010 Dec;25(4):668-674.
DOI: https://doi.org/10.1590/S1983-51752010000400019
3. Zeitoune GC, Subpeitoral ou subglandular: qual é a melhor localização do implante
para pacientes com hipomastia?. Rev Bras Cir Plást. 2012;27(3):428-34. DOI: https://doi.org/10.1590/S1983-51752012000300017
4. Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications,
and patient satisfaction with breast implants. Plast Reconstr Surg. 2006 Mar;117(3):757-67.
DOI: https://doi.org/10.1097/01.prs.0000201457.00772.1d
5. Sperly A, Bersou Júnior A, Freitas JOG, Michalay N. Complicações com próteses mamárias.
Rev Soc Bras Cir Plást. 2000;15(3):33-46.
6. Slavin SA, Greene AK. Augmentation mammoplasty and its complications. In: Thorne CH,
editor. Grabb & Smith’s plastic surgery. Philadelphia: Lippincott Williams & Wilkins;
2007. p.575-584.
7. Tebbetts JB. Dual plane breast augmentation: Optimizing implant-soft-tissue relationships
in a wide range of breast types. Plast Reconstr Surg. 2006 Dec;118(7 Suppl):81S-98S.
DOI: https://doi.org/10.1097/00006534-200612001-00012
8. Teitelbaum S. Abordagem do aumento das mamas em plano duplo. In: Aston SJ, editor.
Cirurgia Plástica Estética. Elsevier. 2011;(54):675-687.
9. Hidalgo DA, Spector JA. Mastopexy. Plast Reconstr Surg. 2013 Oct;132(4):642e-656e.
PMID: 24076713 DOI: https://doi.org/10.1097/PRS.0b013e31829fe4b4
10. De Benito J, Sánchez K. Key points in mastopexy. Aesthetic Plast Surg. 2010 Dec;34(6):711-5.
PMID: 20499062 DOI: https://doi.org/10.1007/s00266-010-9527-5
11. Graf R, Biggs TM. In search of better shape in mastopexy and reduction mammoplasty.
Plast Reconstr Surg. 2002 Jul;110(1):309-17;discussion:318-22. DOI: https://doi.org/10.1097/00006534-200207000-00053
12. Swanson E. A retrospective photometric study of 82 published reports of mastopexy
and breast reduction. Plast Reconstr Surg. 2011 Dec;128(6):1282-301. DOI: https://doi.org/10.1097/PRS.0b013e318230c884
13. Wada A, Millan LS, Gallafrio ST, Gemperli R, Ferreira MC. Tratamento da ptose mamária
e hipomastia utilizando técnica de mamoplastia com pedículo súpero-medial e implante
mamário. Rev Bras Cir Plást. 2012;27(4):576-583. DOI: https://doi.org/10.1590/S1983-51752012000400018
14. Spear SL, Baker Júnior JL. Classification of capsular contracture after prosthetic
breast reconstruction. Plast Reconstr Surg. 1995 Oct;96(5):1119-23;discussion:1124.
DOI: https://doi.org/10.1097/00006534-199510000-00018
15. Meiega JM, Amaral AB, Cunha KN. Arantes HL, Kawasak MC. A Capsulectomia sem Capsulotomia
no Tratamento das Contraturas Capsulares. Rev Bras Cir Plást. 2001;16(2):37-48.
16. Handel N. Secondary mastopexy in the augmented patient: a recipe for disaster. Plast
Reconstr Surg. 2006 Dec;118(7 Suppl):152S-163S;discussion:164S-167S. DOI: https://doi.org/10.1097/01.prs.a0000246106.85435.74
17. Young VL. Guidelines and indications for breast implant capsulectomy. Plast Reconstr
Surg. 1998 Sep;102(3):884-91;discussion:892-4. DOI: https://doi.org/10.1097/00006534-199809010-00043
18. Saraiva JAC. Tratamento das contraturas nas mamoplastias de aumento retroglandulares:
implante retropeitoral com retalho capsular. Rev Bras Cir Plást. 2013 Jul/Sep;28(4):607-610.
19. Tebbetts JB. “Out points” criteria for breast implant removal without replacement
and criteria to minimize reoperations following breast augmentation. Plast Reconstr
Surg. 2004 Oct;114(5):1258-62. PMID: 15457046 DOI: https://doi.org/10.1097/01.PRS.0000136802.91357.CF
20. Spear SL, Carter ME, Ganz JC. The correction of capsular contracture by conversion
to “dual-plane” positioning: technique and outcomes. Plast Reconstr Surg. 2003;112(2):456-66.
PMID: 12900603 DOI: https://doi.org/10.1097/01.PRS.0000070987.15303.1A
21. Virden CP, Dobke MK, Stein P, Parsons CL, Frank DH. Subclinical infection of the silicone
breast implant surface as a possible cause of capsular contracture. Aesthetic Plast
Surg. 1992;16(2):173-9. PMID: 1570781 DOI: https://doi.org/10.1007/BF00450610
22. Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture
in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010 Sep;126(3):835-42.
DOI: https://doi.org/10.1097/PRS.0b013e3181e3b456
23. Spear SL. Capsulotomy, capsulectomy, and implantectomy. Plast Reconstr Surg. 1993;92(2):323-4.
PMID: 8337283 DOI: https://doi.org/10.1097/00006534-199308000-00018
24. Peters W, Smith D, Fornasier V, Lugowski S, Ibanez D. An outcome analysis of 100 women
after explantation of silicone gel breast implants. Ann Plast Surg. 1997 Jul;39(1):9-19.
PMID: 9229086 DOI: https://doi.org/10.1097/00000637-199707000-00002
25. Dinah W, Rohrich RJ. Revisiting the management of capsular contracture in breast augmentation:
a systematic review. Plast Reconstr Surg. 2016 Mar;137(3):826-41. DOI: https://doi.org/10.1097/01.prs.0000480095.23356.ae
26. Mahler D, Hauben DJ. Retromammary versus retropectoral breast augmentation: a comparative
study. Ann Plast Surg. 1982 May;8(5):370-4. DOI: https://doi.org/10.1097/00000637-198205000-00003
1. Centro de Cirurgia Plástica e Bem Estar, Pato Branco, PR, Brazil.
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil.
Corresponding author: Vinicius Julio Camargo Rua Tapir 757, Centro, Pato Branco, PR, Brazil. Zip Code: 85501-032. E-mail: viniciusjcamargo@yahoo.com.br
Article received: January 12, 2019.
Article accepted: July 08, 2019.
Conflicts of interest: none.