

Articles - Year 1999 - Volume 14 -
Doença de Meige (Linfedema Precoce) - Relato de Caso e Revisão de Literatura
Doença de Meige (Linfedema Precoce) - Relato de Caso e Revisão de Literatura
ABSTRACT
Primary lymphedema is a disorder caused bypersistent edema, mainly of extremities and predominating in women. Diagnosis may be made through clinical history, physical examination and supplementary examinations such as lymphangiography, venography and lymphoscintscanning. More frequent complications are recurrent cellulitis and lymphangitis, and rarely lymphangiosarcoma attacks. Treatment ofprimary lymphedema is based on support measures, extremity raising, physical activity, elastic compression; howeven when failure of clinical treatment occurs, surgery is indicated. The case of a 29-year patient with primary lymphedema presenting changes in lymphatic system flow at investigation and being submitted to Charles's procedure associated to early grafting is described. Literature was reviewed.
Keywords: Meige's disease; early lymphedema; Charles's procedure; early partial skin graft
RESUMO
Linfedema primário é um distúrbio causado por edema persistente, principalmente nas extremidades e com predominância em mulheres. O diagnóstico pode ser feito através da história clínica, exame físico e com exames complementares como linfangiografia, venografia e linfocintilografia. As complicações mais freqüentes são ataques recorrentes de celulite e linfangite, e raramente linfangiosarcoma. O tratamento do linfedema primário baseia-se em medidas de apoio, elevação da extremidade, atividade física, compressão elástica; entretanto, na falência do tratamento clínico, está indicada a cirurgia. Descreve-se o caso de uma paciente de 29 anos, com quadro de linfedema primário, que apresentava alterações no fluxo do sistema linfático na investigação, sendo submetida ao procedimento de Charles associado à enxertia precoce. A literatura foi revisada.
Palavras-chave: Doença de Meige; linfedema precoce; procedimento de Charles; enxertia precoce de pele parcial
Lymphedema is a syndrome characterized by hyperproteic fluid accumulation at dermis and tela subcutanea but not at the deep tissues of muscle wall. Etiology includes congenital abnormalities of lymphatic system development or secondary to obstruction, and destruction or malfunction of lymphatic web. Infection, postphlebitic state with venous stasis, local tissue or regional lymphonodes infiltration by malign cells and postlymphadenectomy with or without radiotherapy are among the commonest causes(1,2,3).
CLASSIFICATION AND CLINICAL PRESENTATION
Lymphedema is divided into primary and secondary. Primary or idiopathic lymphedema may be subdivided into congenital form (Milroy's Disease)(4), present at birth; early lymphedema (Meige's Disease)(5), generally appearing from 10 to 35 years old, and late lymphedema, appearing after 35 years old. Early lymphedema comprises approximately 80% of primary lymphedema cases, predominantly in women. When various members of the same family have congenital lymphedema, this pathology has been called Nonne-Milroy-Meige's Disease(6). Onset age and severity tend to be similar in the family(7,8,9).
Familiar-type clinical presentation is not different from non-familiar expression. Most of the patients are women (75%). The more frequently sites involved are legs, arms; genitalia and face may be affected. Edema appears at heels and extends gradually upwards. Lower extremities may be occasionally committed. Trauma occurrence has been described as triggering agent.
Edema is initially soft and disappears with rest but becomes solid with time due to reactive fibrasis complication. Increased temperature, menstruation and pregnancy may stress edema. Skin changed by lymphedema, trauma, fissure, and epidermophytosis permits entry of bacteria, especially hemolytic streptococcus and less frequently staphylococcus, causing recurrent lymphangitis and cellulitis.
Lymphedema pathogenesis is still unknown. Regardless of lymphedema primary cause (malformation or obstruction), edema occurs when capillary filtration exceeds lymphatic and venous absorption. Lymphedema initial phase is reversible. Infections and neoplasias are the major lymphedema complications. Rich with protein edema serves as an excellent medium for bacterial growth. These patients will inevitably develop cellulitis and lymphangitis. Approximately 25% of the patients experience inevitable episodes of lymphangitis. Therefore, longterm aggressive therapy, with low dose oral antibiotics for staphylococcus and streptococcus infections may be beneficial.
DIAGNOSIS
Lymphedema diagnosis is mainly clinical, based on history, physical examination and exclusion of other possibilities. Extremity involved is usually painful, with no functional ability, edematous, cold and bluish.
Several diagnostic methods may be useful: lymphangiography, which is not routinely carried out due to technical difficulties and complications, and lymphoscintscanning, which is safe, reliable and became the method of choice for visualizing and evaluating lymphatic function. Other diagnostic methods include Doppler ultrasound, computerized axial tomography and nuclear magnetic resonance(9,10,11,12,13,14,15).
DIFFERENTIAL DIAGNOSIS
Lymphedema differential diagnosis includes common shapes of bilateral edema (such as nephrosis, congestive heart failure, cyclic or idiopathic edema, hypoproteinemia states and myxedema) and unilateral edematous conditions (such as venous failure, angiodermatitis and lipedema). Clinical finding of non-depressive, solid edema favors lymphedematous origin but biopsy associated to lymphangiogram and venogram may be required for a definite diagnosis(7).
COMPLICATIONS
Main lymphedema complications are recurrent episodes of cellulitis and lymphangitis. Development of lymphangiosarcoma is a rare complication that may arise from congenital lymphedema(7).
TREATMENT
Lymphedema is an incurable condition in terms of total disappearance of edema and extremity contour recovery. Clinical treatment with mechanical support or medications, or both, may be satisfactory for most lymphedemic patients.
Lymphedema treatment aims at maintaining tissues free from edema to prevent fibrosis and recurrent bacterial infections. Weight loss, frequent raising of extremity involved, use of graded elastic mesh, physiotherapy, diuretics and a poor-salty diet are routine therapeutic measures. External pneumatic compression pump or intermittent sequential compression followed by daily use of graded elastic mesh at committed extremity may be useful to reduce lymphedema.
Epidermophytosis should be vigorously treated and controlled. Long-term intermittent antibiotic prophylaxis is recommended to recurrent lymphangitis.
Factors that worsen edema include excessive use of limb, local heat, increased room temperature and injuries by sharp objects. Ulceration is uncommon and usually follows a local trauma.
SURGICAL TREATMENT
Surgical treatment is the most controversial area. Charles proposed one of the first excision techniques in 1912, which comprised skin and tela subcutanea removal down to muscle fascia, presenting relatively good results, reducing extremity size and leaving, however, a residual scar and an ungraceful postoperative appearance(7,10,17). Thompson (1960) developed an embellished dermal flap to improve subcutaneous tissues lymphatic drainage to deep lymphatic trunks. Initial results were encouraging but lately investigators showed that operation success was caused by lymphatic tissue removal only(18). Goldsmith and cols. developed a transposition technique of rich with lymphatic tissue omentum to drain the extremity involved. Results of this operation have been poor(19). Microsurgery to create venous-lymphatic anastomosis has been used nowadays in the attempt to bypass obstructions of lymphatic system(17).
Surgical procedure is recommended for patients with non-controlled edema, excessive deformity and mobility reduction due to extremity size. Patient needs to be hospitalized, and must keep extremity raised for several days to eliminate all residual edema before operation. If necessary, a pneumatic pump may be used. Surgical treatment is not indicated to children younger than 2 years old as occasionally edema may decrease upon deambulation start. Results of surgical approach to extremity are less satisfactory than expected, however, in genital operations, surgical treatment has obtained good results(3).
In short, extremity surgery should be used as last resource when conservative measures fail. Early surgery in children and teenagers should be avoided due to the high variability of the disease course. If surgery is indicated, patient should be warned that success of this procedures is only 30% and that complications, specifically the cosmetic ones, are frequent. A discouraging fact remains: no procedure cures lymphedema(17,8).
CASE REPORT
Female patient, 29 years old, born in Curitiba - PR, with superficial thrombosis picrure at left lower lirnb for 11 years, associated to erysipelas. When submitted to clinical treatment with venodilating, anti-inflammatory and antibiotic drugs, she achieved relative improvement with persistem ankle edema. One year after this picrure, phlebography was carried out with no abnormalities. Nine years ago she presemed major edema at left thigh root associated to recurrent picrures of erysipelas. During this period she wore high-compression elastic stockings and external pneumatic compressor, achieving edema reduction.
At first pregnancy, 4 years ago, she presented increased volume of affected limb (Fig. 1). Next year, she was subrnitted to lymphovenous shunt surgery at inguinal region, with thigh volume decrease. In November in the same year, surgery with embellished flap (Thompson - Fig. 2) was carried out with worsening of clinicaI picture and resulting in functional disability, stressed after second pregnancy, two years after thefirst one. One year ago, lymphoscintscanning of lower limbs was carried out which showed absence of lymphatic vessels at left lower limbo Patient then was submitted to Charles's surgery (Figs. 4, 6 and 7) except for knee joint area and thigh root, aiming at preserving sound skin. The surgical piece weighted approximately 40 kg (Fig. 5). Immediate grafting was carried out at partial skin, piece was drawn with Blair's knife (Fig. 3) ad prepared with Tanner's expander (Fig. 8).
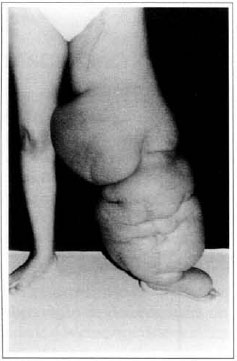
Fig. 1 - Primary lymphedema.
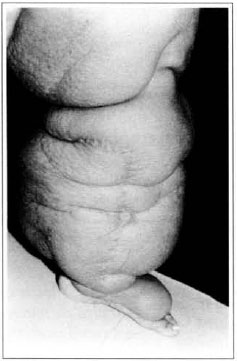
Fig. 2 - "Peau d'orange" aspect and Thompson surgery scar.
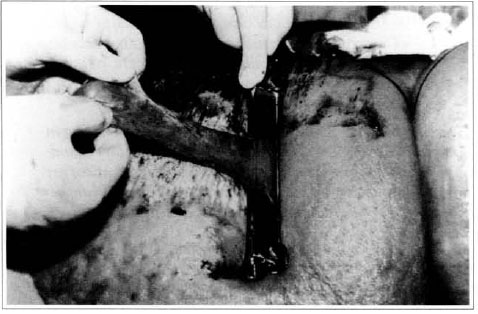
Fig. 3 - Graft drawing of partial skin graft.
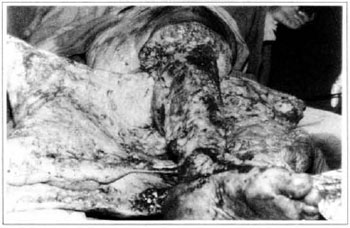
Fig. 4 - Charles's surgery.
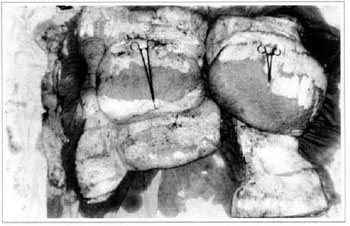
Fig. 5 - Surgical piece.
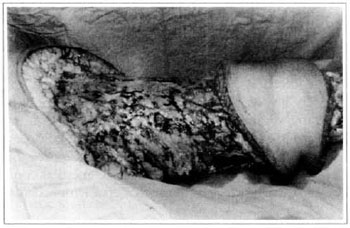
Fig. 6 - Charles's surgery - transoperative period.
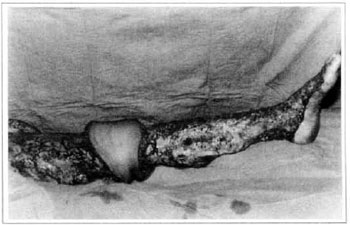
Fig. 7 - Charles's surgery - transoperative period.
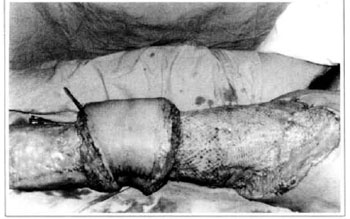
Fig. 8 - Immediate aspect of skin graft - thigh mesh-graft.
Patient remained in hospital for a 30-day period, being submitted to occlusive dressings each 2 day with topical polvidine, isotonic saline, petrolaturn rayon and topical rifamycin, remaining at room with lower limb raised. Systemic antibiotic therapy was also administered. Patient was submitted to motor physiotherapy for 20 days after procedure, being discharged deambulating, with integrated graft (Figs. 9 and 10) and return scheduled for knee joint lipoaspiration. After 60 days, patient was submitted to"a new surgical procedure to decrease skin diameter and tela subcutanea at knee joint region associated to lipoaspiration (Fig. 11), using elastic stockings subsequently. She remained stable with lower limb volume maintained for 3 months. Edema relapsed at knee joint region probably because patient did not use elastic stockings and so skin and tela subcutanea and immediate partial skin graft were chosen. After 6 months from this last procedure, patient is stable with no relapses or residual edema (Figs. 12 and 13), requiring thigh root lymphedema correction only.
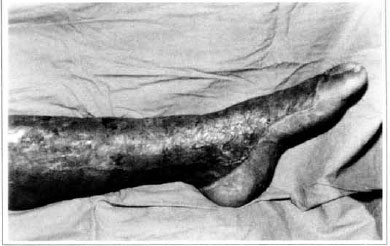
Fig. 9 - Imegrated graft.
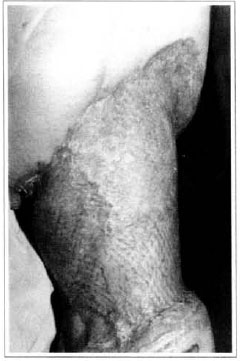
Fig. 10 - Final aspect of mesh-graft - residual volume at thigh.
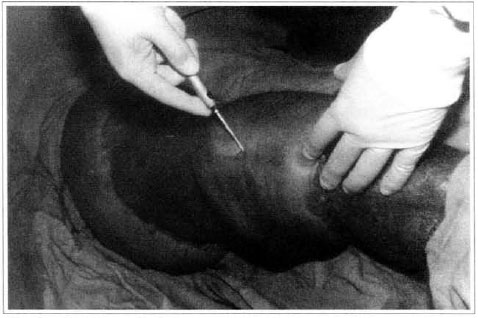
Fig. 11 - Knee lipoaspiration.
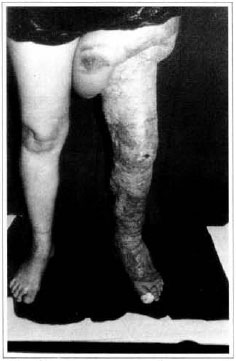
Fig. 12 - Late postoperative aspeet, 6 months - anterior view.
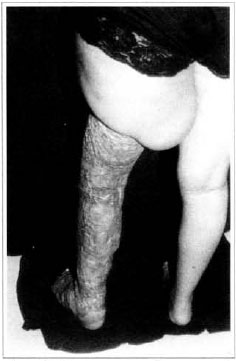
Fig. 13 - Late posroperarive aspect, 6 months - posterior view.
REFERENCES
1. ARAÚJO JA, CURBELO JG. Effective management of marked lymphedema of the leg. Int. J. Derm, 1997; 36:389-392.
2. MORTIMER P, REGNARD C. Lymphostatic disorders. Br. J. Surg. 1986; 293:347-348.
3. MOREY AF, MENG MV, McANINCH JW. Skin graft reconstruction of chronic genital lymphedema. Urology. 1997; 50(3):423-6.
4. MILROY WF. Chronic hereditary edema: Milroy's disease. JAMA. 1928; 91:1172- 1174.
5. MEIGE H. Dystrophie oedémateuse héréditaire. Presse Med. 1898; 2:341-343.
6. NONNE M. Vier Falie von Elephantiasis congenita hereditaria. Arch. Pathol. Anat. Physiol. 1891; 125:189-196.
7. KINMONTH JB. Primary lymphedema of the lower limb. Proc. R SOC. Med. 1965; 58:1021-1025.
8. KINMONTH JB, TAYLOR cw TRACY GD, MARSH JD. Primary lymphedema. Br. J. Surg. 1957; 65:3-9.
9. WOLFE JHN, KINMONTH JB. The prognosis of primary lymphedema of the lower limbs. Arch. Surg. 1981; 116:1157-1160.
10. HAREL L, AMIR J. Lymphedema praecox seen as isolated unilateral arm involvement: Case report and review of the literature. J. Pedias. 1997; 130:492-494.
11. PROBY CM, GANE JN. Investigation ofthe swollen limb with isotope lymphography. Br. J. Derm. 1990; 123:29-37.
12. GOUGH MH. Primary lymphoedema: Clinical and lymphangiographic studies. Br. J. Surg. 1966; 53:917-925.
13. DUEWEL S, HAGSPIEL KD. Swollen lower extrerniry: Role of MR Imaging. Radiology. 1992; 184:227-231.
14. WEISSLEDER H, WEISSLEDER R. Lymphedema: Evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology. 1988; 167:729-735.
15. HARWOOD CA, BULL RH, EVANS J, MORTIMER PS. Lymphatic and venous function in lipoedema. Br. J. Surg. 1996; 134:1-6.
16. CHARLES H. A system of treatment. Latham & English) 1912. Voll, p. 123-147.
17. SMELTZER DM, STICKLER GB, SCHIRGERA. Primary lymphedema in children and adolescents: A follow-up study and review. Pediatrics. 1985; 76:206-217.
18. THOMPSON N. Ibid. 1962; 2:1566.
19. GOLDSMITH HS, DE LOS SANTOS R, BEATTIE EJ. Relief of chronic lymphedema by omental transposition. Ann. Surg. 1967; 166:573-585.
I - Resident doctor of Plastic Surgery.
II - Senior member of SBCP. Department of Plastic Surgery and Burns of Hospital Evangélico de Curitiba.
III - Vascular surgeon. Department of Vascular surgery of Hospital Evangélico de Curitiba.
IV - Medical student.
Address for correspondence:
Ronaldo Roesler, MD
R. Aristides Athayde Jr., 560 - apto. 171
80730-370 - Curitiba - PR Brazil
Phone: (5541) 336-8178


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter