ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Report of a case of anaplastic large cell lymphoma associated with a breast implant in a Brazilian patient
Relato de caso de linfoma anaplásico de células grandes associado a implante mamário em paciente brasileira
Case Report -
Year2017 -
Volume32 -
Issue
3
Bernardo Nogueira Batista1; Bernardo Garicochea1; Vera Lucia Nunes Aguilar2; Filomena Marino Carvalho3; Lincoln Saito Millan4; Murillo Francisco Pires Fraga1; Marcelo Moura Costa Sampaio1; Alfredo Carlos Simões Dornellas Barros1
http://www.dx.doi.org/10.5935/2177-1235.2017RBCP0073
ABSTRACT
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a T-cell lymphoproliferative disorder that has recently been recognized as an independent entity in the World Health Organization (WHO) classification of lymphomas. Despite the small number of reports to date, the number of cases is rapidly increasing. Of the few hundred cases that have been reported so far, very few came from Brazil and none have been reported to the local authorities. We encountered a case of BIA-ALCL and believe that its report to the local plastic surgery community could raise awareness to this emerging pathology. The prognosis is very good in most of the diagnosed cases. However, little is known about how and why silicone implants could trigger a lymphoid response that results in ALCL.
Keywords:
Lymphoma large-cell anaplastic; Breast implants; Breast neoplasms; Mammoplasty; Seroma.
RESUMO
O linfoma anaplásico de células grandes (ALCL) associado a implantes mamários é um distúrbio linfoproliferativo das células T que foi recentemente reconhecido como uma entidade independente na classificação de linfomas da Organização Mundial de Saúde (OMS). Apesar do pequeno número de descrições, o número de casos está crescendo rapidamente. Das poucas centenas de casos que foram publicados até agora, muito poucos vieram do Brasil e nenhum foi relatado às autoridades locais. Encontramos um caso recentemente, e acreditamos que seu relato à comunidade local de cirurgia plástica poderá chamar a sua atenção para essa patologia emergente. O prognóstico é muito bom na maior parte dos casos diagnosticados. Contudo, ainda se sabe pouco sobre como e por que os implantes de silicone poderiam desencadear uma resposta linfoide, culminando num ALCL.
Palavras-chave:
Linfoma anaplásico de células grandes; Implantes de mama; Neoplasias da mama; Mamoplastia; Seroma.
INTRODUCTION
Plastic surgeons worldwide have been surprised by the increasing number of women diagnosed with a new type of lymphoma that occurs in the presence of breast implants; the first such case was reported in 19971. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) has recently been included in the World Health Organization classification of lymphoid neoplasms2 and remains a concern in modern plastic surgery practices3. Recent efforts to improve reporting and data analysis have been undertaken by regulatory agencies and medical societies globally4-6.
Although Brazil is the second largest market for breast implants worldwide, no cases of BIA-ALCL have been officially reported to the Agência Nacional de Vigilância Sanitária (ANVISA) so far7. We encountered a case of BIA-ALCL recently and believe that its report will increase awareness of ALCL within the local plastic and breast surgery community.
CASE REPORT
A 44-year-old female patient presented to our clinic complaining of mild discomfort in her right breast for almost a year and recent enlargement of the same breast. The patient was otherwise healthy. She had undergone a primary periareolar breast augmentation with 195-cc bilateral polyurethane-coated implants 11 years previously.
There was marked volume asymmetry with discrete skin infiltration in the lower medial aspect of the breast (Figure 1). Physical examination and imaging revealed a 10-cm heterogeneous mass posterior to the implant that infiltrated the pectoralis major muscle (Figure 2). There was no seroma. The initial suspicion of BIA-ALCL was confirmed using an ultrasound-guided biopsy and pathological studies (CD30+; ALK-) (Figure 3)4.
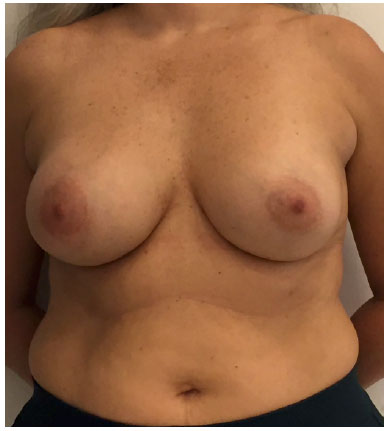 Figure 1.
Figure 1. Preoperative frontal view.
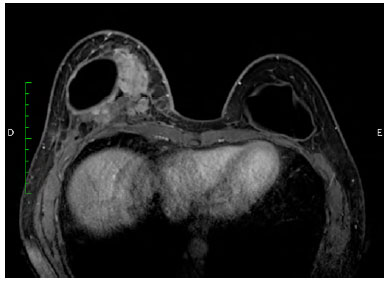 Figure 2.
Figure 2. Magnetic resonance image (transversal plane), showing a heterogeneous mass medially, posterior to the implant.
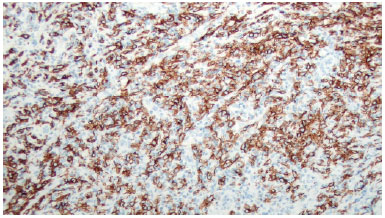 Figure 3.
Figure 3. Immunohistochemical CD30 staining.
A pre-operative positron emission tomography (PET) scan showed an increased glucose metabolism in the breast area (SUV = 9.5) and in an axillary lymph node. This node had been biopsied a few weeks earlier and no malignancy was detected (SUV = 2.0) (Figure 4A). Surgical planning and adjuvant therapy were discussed by a multidisciplinary team and with the patient, based on the best evidence available at the time.
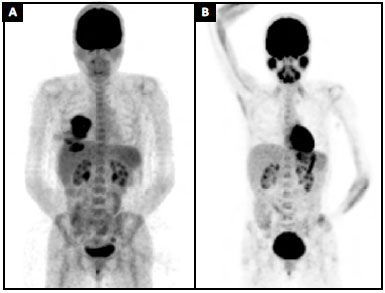 Figure 4. A:
Figure 4. A: Preoperative positron emission tomography (PET) scan (MIP) showing an increase in glucose metabolism in the breast area (SUV = 9.5);
B: Nine months' follow-up positron emission tomography (PET) scan (MIP).
The surgical procedure involved removal of the implant, total capsulectomy including the mass, and removal of all macroscopically suspicious tissue (Figure 5). This resection included the pectoralis major muscle and part of the breast tissue and skin from the medial lower pole that had an abnormal appearance at inspection (Figure 6). Reconstruction was achieved with an oncoplastic mammoplasty (Figure 7).
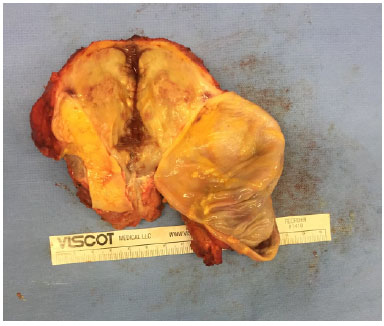 Figure 5.
Figure 5. Surgical specimen from the right breast.
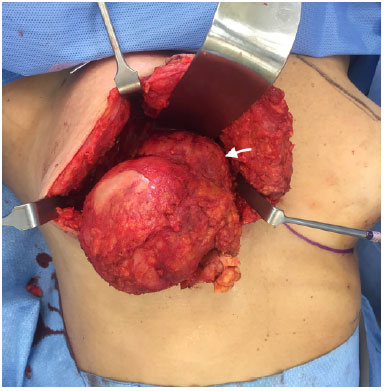 Figure 6.
Figure 6. Macroscopic aspect after capsulectomy and tumor (arrow) dissection. The mass is infiltrating the pectoralis major muscle.
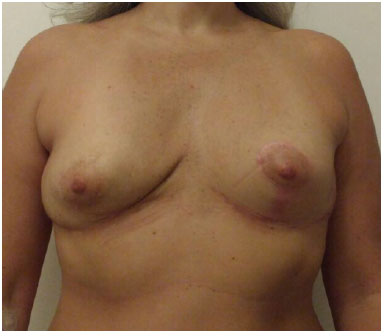 Figure 7.
Figure 7. Nine months post-operatively, frontal view.
The implant shell and polyurethane coating were intact. The right axilla was opened and manually explored for hard or enlarged nodes; however, none were found. Contralateral implant removal and capsulectomy were performed alongside conventional mastopexy. Pathological examination of the specimens from the right breast revealed infiltration of pleomorphic aberrant cells in the necrotic mass, adjacent muscle, breast tissue, and skin without a clear limit and positive margins. Additional skin removed for final shaping of the breast was clear of the aberrant lymphocytes; however, negative margins could not be guaranteed.
The patient chose not to receive any adjuvant treatment. At the time, we did not feel that there was enough evidence to indicate any benefit from chemotherapy, and the patient refused radiotherapy. Nine months post-operatively, her PET scan shows complete remission of the disease (Figure 4B). This case was registered in the PROFILE Registry (http://www.thepsf.org/research/clinical-impact/profile.htm).
DISCUSSION
BIA-ALCL is a very rare complication of breast implants. However, given the number of Brazilian women with breast implants, every plastic and breast surgeon may encounter this emerging lymphoma. We still know very little about BIA-ALCL and the current evidence is limited to case reports and cross-sectional studies from the American, Australian, and British task forces. So far, a few hundred cases have been reported.
A study from the USA estimated the lifetime prevalence of BIA-ALCL to be 1 per 30,000 women with a textured breast implant4. BIA-ALCL has been reported in association with all types of textured implants; however, the Australian task force has suggested that implants from certain manufacturers present a much higher risk than others5.
Late seroma has been the most frequently reported presentation of BIA-ALCL. However, late seromas in patients with breast implants are much more likely to be benign. Awareness of BIA-ALCL is important since diagnoses can only be made using specific immunohistochemistry studies of the fluid around the implant (CD30 and ALK staining).
Pathologists should be informed of the clinical suspicion and the specific tests required, as these are not routinely performed on specimens from the breast. In our case, the lymphoma presented as a mass, another presentation of the disease that has been reported. It is still unclear if these are different forms of the disease or part of a disease spectrum; however, the mass form of the disease has been associated with a poorer prognosis3,5,6.
Removal of the implant with total capsulectomy seems to be sufficient to treat BIA-ALCL. Adjuvant radiotherapy and chemotherapy have been used in some of these patients, without a clear benefit. A recent guideline from the National Comprehensive Cancer Network proposed adjuvant treatment for BIA-ALCL not confined to the seroma fluid and capsule8.
However, these adjuvant therapies have acute and lifelong morbidity that must be considered and discussed with the patient. Most of the current recommendations are still based on preliminary data and it will be a long time before we have solid evidence to guide clinical decision-making in patients presenting with BIA-ALCL.
In our case, the patient was very resistant to traditional oncological care and decided not to undergo radiation after multidisciplinary discussions. The follow-up period is short, although the patient is currently in remission. Very few deaths have occurred in patients with BIA-ALCL; mortality is believed to as low as 2.5%. Since the severity of clinical presentation and prior clinical suspicion are important sources of bias for reporting, this estimation is probably inflated.
The impact of this new entity on the breast surgery community is still unclear. The reactions of the public and the media to BIA-ALCL will depend on our efforts to generate information and to communicate properly.
ACKNOWLEDGEMENTS
We acknowledge Drs. Edward I-Fei Chang and Mark W. Clemens for their availability and support during the management of the case reported here.
COLLABORATIONS
BNB Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
BG Final approval of the manuscript; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
VLNA Final approval of the manuscript; completion of surgeries and/or experiments.
FMC Final approval of the manuscript; completion of surgeries and/or experiments.
LSM Completion of surgeries and/or experiments; writing the manuscript or critical review of its contents.
MFPF Conception and design of the study; writing the manuscript or critical review of its contents.
MMCS Final approval of the manuscript; conception and design of the study; writing the manuscript or critical review of its contents.
ACSDB Final approval of the manuscript; conception and design of the study.
REFERENCES
1. Keech JA Jr., Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100(2):554-5. PMID: 9252643
2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-90. PMID: 26980727 DOI: http://dx.doi.org/10.1182/blood-2016-01-643569
3. Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, Andersen JS, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135(3):695-705. Erratum in: Plast Reconstr Surg. 2015;136(2):426.
4. Doren EL, Miranda RN, Selber JC, Garvey PB, Liu J, Medeiros LJ, et al. U.S. Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg. 2017;139(5):1042-50. DOI: http://dx.doi.org/10.1097/PRS.0000000000003282
5. Loch-Wilkinson A, Beath K, Knight RJW, Wessels WLF, Magnusson M, Papadopoulos T, et al. Breast implant associated Anaplastic Large Cell Lymphoma in Australia and New Zealand - high surface area textured implants are associated with increased risk. Plast Reconstr Surg. 2017. DOI: 10.1097/PRS.0000000000003654. [Epub ahead of print] DOI: http://dx.doi.org/10.1097/PRS.0000000000003654
6. Johnson L, O'Donoghue JM, McLean N, Turton P, Khan AA, Turner SD, et al. Breast implant associated anaplastic large cell lymphoma: The UK experience. Recommendations on its management and implications for informed consent. Eur J Surg Oncol. 2017;43(8):1393-401. DOI: http://dx.doi.org/10.1016/j.ejso.2017.05.004
7. Srinivasa DR, Miranda RN, Kaura A, Francis AM, Campanale A, Boldrini R, et al. Global Adverse Event Reports of Breast Implant-Associated ALCL: An International Review of 40 Government Authority Databases. Plast Reconstr Surg. 2017;139(5):1029-39. DOI: http://dx.doi.org/10.1097/PRS.0000000000003233
8. Clemens MW, Horwitz SM. NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet Surg J. 2017;37(3):285-9. DOI: http://dx.doi.org/10.1093/asj/sjw259
1. Hospital Sírio Libanês, São Paulo, SP, Brazil
2. Laboratório Fleury, São Paulo, SP, Brazil
3. Faculdade de Medicina, Universidade de São Paulo, SP, Brazil
4. Breast Academy, Gold Coast, Australia.
Institution: Hospital Sírio Libanês, São Paulo, Brazil.
Corresponding author:
Bernardo Nogueira Batista
Praça Amadeu Amaral, 27 - 6º andar
São Paulo, SP, Brazil - Zip Code 01327-010
E-mail: bernardonb@uol.com.br
Article received: July 21, 2017.
Article accepted: August 17, 2017.
Conflicts of interest: none.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license














