ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Cutaneous approach in toxic epidermal necrolysis
Abordagem cutânea na necrólise epidérmica tóxica
Review Article -
Year2017 -
Volume32 -
Issue
1
Francisco Carlos Santos Neto1; Pedro Salomão Piccinini1; Jean Miguel Andary1; Lucas Dal Pozzo Sartori1; Lucas Tomkowski Cancian1; Carlos Oscar Uebel1,2,3; Milton Paulo de Oliveira1,2
http://www.dx.doi.org/10.5935/2177-1235.2017RBCP0018
ABSTRACT
INTRODUCTION: Toxic epidermal necrolysis is a severe acute mucocutaneous condition usually induced by drugs associated with a high rate of morbidity and mortality. The care of the mucous lesions and skin and a multidisciplinary approach are very important for the prognosis and future sequelae.
OBJECTIVES: To discuss the main aspects of this syndrome through a literature review illustrated by a clinical case. Methods: Review of the literature using the PubMed and SciELO online databases was performed. Articles in English, Portuguese, French, and Spanish were included and illustrated with a pediatric clinical case. The keywords used were as follows: "toxic epidermal necrolysis," "Stevens-Johnson overlap," "necrólise epidérmica tóxica," and "síndrome Stevens-Johnson."
RESULTS: We presented data to guide the management of patients with toxic epidermal necrolysis for plastic surgeons, pediatricians, intensivists, dermatologists, and emergency physicians. The case treated had a favorable disease course without sequelae.
CONCLUSION: A high level of suspicion is necessary for an adequate diagnosis and risk stratification, and early support measures and treatment should be performed by a multidisciplinary team trained to minimize damage and mortality.
Keywords:
Dermatology; Exanthema; Stevens-Johnson Syndrome; Surgical Procedures; Child.
RESUMO
INTRODUÇÃO: Necrólise epidérmica tóxica é uma erupção mucocutânea aguda grave, geralmente induzida por medicamentos, associada a alta taxa de morbidade e mortalidade. Os cuidados com as lesões mucosas e cutâneas e a abordagem multidisciplinar são muito importantes para o prognóstico e sequelas futuras.
OBJETIVOS: Discutir os principais aspectos dessa síndrome por meio da revisão de literatura, ilustrada por um caso clínico.
MÉTODOS: Revisão de literatura utilizando bases de dados on-line PubMed e Scielo. Incluímos artigos em língua inglesa, portuguesa, francesa e espanhola, e ilustração com caso clínico pediátrico. Termos procurados foram "toxic epidermal necrolysis", "Stevens-Johnson overlap", ''necrólise epidérmica tóxica'', ''síndrome Stevens-Johnson''.
RESULTADOS: Apresentamos dados para guiar o manejo de pacientes com necrólise epidérmica tóxica para cirurgiões plásticos, pediatras, intensivistas, dermatologistas e emergencistas. O caso tratado teve evolução favorável, sem sequelas cutâneas.
CONCLUSÃO: O alto nível de suspeição é imprescindível para um diagnóstico e estratificação de risco adequados e instituição precoce de medidas de suporte, e o tratamento deve ser realizado por uma equipe multidisciplinar treinada para reduzir sequelas e mortalidade.
Palavras-chave:
Dermatologia; Exantema; Síndrome de Stevens-Johnson; Procedimentos cirúrgicos operatórios; Criança.
INTRODUCTION
Toxic epidermal necrolysis (TEN) is a severe acute mucocutaneous eruption usually induced by drugs associated with a high morbidity and mortality rate1.
The death of keratinocytes in the dermal-epidermal junction usually begins between one and three weeks after exposure to drugs and can be preceded by viral prodromes, such as fever and myalgia2. Care of the mucous lesions and skin and a multidisciplinary approach are very important for the prognosis and prevention of future sequelae2.
In this article, we present an updated literature review on healthcare in TEN focused on skin care. With an adequate stratification and supportive care, a reduction in its morbidity and mortality is possible.
OBJECTIVES
To discuss the main aspects of TEN through a literature review illustrated by a clinical case in a pediatric patient, stressing the need for risk stratification, early supportive care in the intensive care unit (ICU) (preferably in a specialized burn center), and management by a multidisciplinary team as strategies to achieve better outcomes in these patients.
METHODS
A review of the literature was performed using the online databases PubMed and SciELO. Articles in English, Portuguese, French, and Spanish were included and illustrated with a pediatric clinical case. The keywords used were as follows: "toxic epidermal necrolysis," "Stevens-Johnson overlap," "necrólise epidérmica tóxica," and "síndrome Stevens-Johnson."
CASE REPORT
A 10-month-old male white patient suspected with Stevens-Johnson Syndrome (SJS) was referred to a community hospital. There was no history of comorbidities, and the pregnancy had occurred without complications. There was no family history of allergic reactions to medications or clinical picture of SJS/TEN. On arrival, the mother of the patient reported a history of approximately one month of symptoms, such as upper respiratory tract infections (URTI), runny nose, mild fever, and cough. The patient had been treated by a pediatrician with ibuprofen, paracetamol, hydroxyzine, and finally azithromycin. He had perioral and malar region erythema (Figure 1) as well as a fever of 38.6ºC.
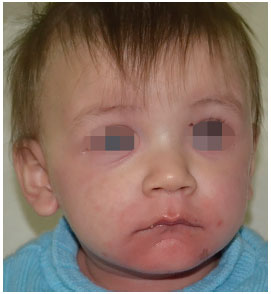 Figure 1.
Figure 1. Initiation of skin lesions on the face.
He was referred to Hospital São Lucas of Pontifical Catholic University of Rio Grande do Sul (PUC/RS), in Porto Alegre, RS. With worsening of the symptoms, the patient was transferred by air to the pediatric ICU at our institution on the second day of hospitalization. All cultures had negative findings. Anti-SSA serologies, anti-DNA antibodies, anti-Sm, rheumatoid factor, antinuclear autoantibodies, toxoplasmosis IgM and IgG, total hemolytic complement, antiparvovirus B19 IgG and IgM antibodies, and rotavirus antibodies were all negative. Antibodies against herpes IgG (6.26), IgG against CMV (73), and IgG against EBV (10.46) were positive.
The Plastic Surgery service of PUC/RS led the care of the lesions. Upon the initial assessment of the team, widespread erythematous, scaly, and blistering lesions were present, affecting ~90% of the total body surface (TBS) area, including the oral mucosa and glans (Figures 2-4). The ocular conjunctiva was spared. There were no signs of infection in the skin. The histopathologic report revealed acanthosis, discreet, sparse necrotic keratinocytes, ortho- and parakeratosis, minimal superficial perivascular lymphocytic infiltrate, and absence of bullous lesions in the sample.
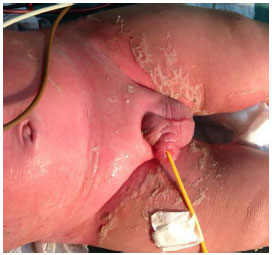 Figure 2.
Figure 2. Involvement of the skin and genital mucosa with skin desquamation.
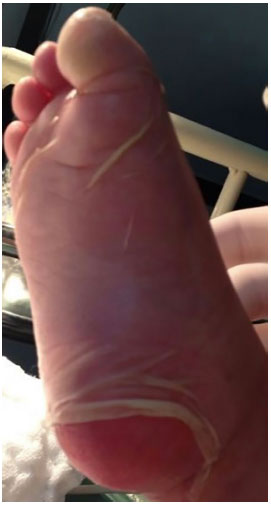 Figure 3.
Figure 3. Nikolsky's sign (dermis-epidermal disjunction) in the plantar region.
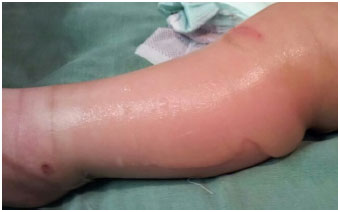 Figure 4.
Figure 4. Features of the bullous lesions in the lower limb.
On the basis of the presentation, bandages with petroleum gauze covered with absorbent cotton dressings were applied. The dressing changes were scheduled every 48 hours under sedation with an abundant washing of wounds and new covering with dressings until complete reepithelialization. There were no signs of infection on the skin during the disease course.
RESULTS
The early and well-conducted care led to a favorable outcome of the case, even with a high rate of mortality for this extension of cutaneous involvement. There were no sequelae, scars, or skin pigmentation changes that caused stigma to the patient.
DISCUSSION
In 1956, Alan Lyell coined the term necrolysis to describe four patients who had a pathologic diagnosis of necrosis of the epidermis characterized clinically by disseminated epidermolysis, a peeling of the epidermis with subsequent blistering, exfoliation, or both. Wide areas of the skin had peeled off, leaving a raw undressed base. A short-lived toxic fever reaction associated with a scalded skin appearance had preceded epidermal necrolysis.
Lyell coined the condition as TEN and suspected that there was a specific circulating toxin that damaged the epidermis1,3. The characteristic pathological finding is epidermal desquamation by apoptosis of keratinocytes2,3. Serum levels of tumor necrosis factor-alpha, interleukin (IL)-2, IL-6, and C-reactive protein are generally elevated but are not routinely used in the diagnosis of SJS4.
The separation of the epidermis from the dermis results in the formation of blisters and peeling of the skin5. Although rare, with an incidence of 2 per million per year6,7, it presents a high mortality of ~30%7,8. The condition is more common in patients with HIV-1 and systemic lupus erythematosus and patients undergoing bone marrow transplant1,4,5.
In the spectrum of scaly dermatological diseases, SJS is defined by lesions in up to 10% of the TBS, with a mortality rate of 1-5%; the SJS-TEN overlap syndrome is defined when there is flaking of >10-30% of the TBS and TEN when >30% of the TBS is involved with a mortality rate of 25-30%. The most common cause of death is sepsis with multiple organ failure, with additional morbidities related to gastrointestinal hemorrhage, pulmonary embolism, acute myocardial infarction, and pulmonary edema9-11.
The drugs most implicated in the etiopathogenesis of TEN are as follows: allopurinol, aromatic anticonvulsants, sulfonamide antibiotics, and non-steroidal anti-inflammatory drugs5. The exposure to these drugs usually precedes the symptoms by one to two weeks but can be as short as 48 hours12-14.
These drugs can stimulate the immune system by binding directly to the major histocompatibility complex (MHC) and receptors in the T-cells, resulting in a specific population of cytotoxic T-cells directed to kill the keratinocytes directly and indirectly12.
Multiple different human leukocyte antigen (HLA) alleles have been associated with an increased risk of developing TEN when patients are exposed to different drugs. The medication more strongly associated with TEN is carbamazepine9,15 to the point that the Food and Drug Administration (FDA) in the United States recommended a genetic screening for all patients prescribed with carbamazepine9.
Clinically, the syndrome presents with fever and influenza symptoms one to three days before the development of mucocutaneous lesions. Skin lesions begin as poorly defined coalescing macules or diffuse erythema. Lesions begin on the face and chest before they spread to the rest of the body and are usually symmetrical. Further, the Nikolsky's sign (detachment of the skin with slight friction) may also be present5,16. Another characteristic sign is the Asboe-Hansen sign (a slight pressure on a blister causes lateral detachment of the epidermis)7.
Lesions of the oral mucosa and vermilion are very frequently present and may precede skin lesions. Ocular involvement occurs in >80% of cases, and conjunctivitis with purulent discharge is the most frequent manifestation. Urethritis can develop in up to 2/3 of cases, causing urinary retention5,7.
The definitive diagnosis depends on the histopathological examination of the skin biopsy, which shows epidermal necrosis and a mild or absent infiltration in the papillary dermis, composed mainly of lymphocytes and macrophages3,8. The exact pathogenesis is still unclear; however, some points in the sequence of molecular and cellular events that lead to NET have been elucidated.
The lesions contain CD8 T-lymphocytes specific for each medication, which exhibit cytotoxicity against keratinocytes17,18. The cytotoxic effects of these CD8 T-cells are mediated by various soluble mediators, such as granulysin and other compounds. The low cellularity of the dermal infiltrate compared to other inflammatory diseases points to a possible role of mechanisms, such as indirect apoptosis of keratinocytes via specific molecular contacts14,15.
A scale was developed to assess the severity of the disease and prediction of mortality in patients with acute TEN: Severity of Illness Score for Toxic Epidermal Necrolysis (SCORTEN). This scale assigns a point for each of seven variables: (1) age >40 years; (2) heart rate (HR) >120 beats per minute (bpm); (3) associated malignant neoplasm; (4) epidermal detachment of >10% of the TBS in the first day; (5) urea >28 mg/dL; (6) glucose >252 mg/dL; and (7) sodium bicarbonate <20 mEq/L. The mortality increases by 3.2% in a patient with 0-1 points, 35.3% with 3 points, and 90% with >5 points. The accuracy of SCORTEN in predicting the mortality has already been validated in other studies; the test should be applied on post-hospitalization days 1 and 3 to optimize its predictive value9-11,19.
There is no gold standard for the treatment of wounds. Patients should be referred to a burn center. The devitalized epidermis should be removed and aggressive debridement avoided. Non-adherent, biological, or synthetic dressings are used6,7.
The wound should be monitored for infection, since its appearance is common and is a major cause of mortality in these patients. The most common bacteria are the gram-positive bacteria, such as Staphylococcus aureus. Prophylaxis with antibiotics showed no improvement in survival19,20 and should not be held.
Patients who survive the acute phase have a reduced survival, with one study showing a survival rate of 65% in 5 years. In this analysis, predictive factors for the increased risk of death after hospital discharge included older age, SCORTEN score between 3 and 6, >1 comorbidity, delay >5 days between the beginning of TEN and hospitalization in a burn unit, and greater involvement of the TBS21.
In a study that examined the histological characteristics of TEN, density of the dermal infiltrate, and severity of epidermal necrosis as possible predictors of mortality, there was no correlation with increased mortality during initial hospitalization6,8. The serum level of bicarbonate <20 mmol/L was associated with a 40-fold higher mortality rate in patients, with epidermal desquamation >30% of the TBS being the best predictor of mortality in TEN20,22.
Survivors of TEN have a high risk of sequelae, including scars, eye lesions, depigmentation, and dental, genitourinary, and lung disease complications. Ocular complications, including sicca syndrome, photophobia, sandy foreign body sensation in the eye, symblepharon, corneal scars and xerosis, blindness, visual acuity reduction, and subconjunctival fibrosis, are the most common, occurring in 20-84% of patients. Dry eye syndrome is the most common ocular complication and may occur even in patients without acute ocular involvements.
Thus, an early ophthalmologic consultation is recommended in all cases of TEN, especially in patients younger than 18 years given the worse ophthalmic outcomes observed in these patients6,23-25. The SCORTEN score is not related to the severity of ocular involvement, and the use of immunomodulating therapy, such as systemic corticosteroids, intravenous immunoglobulin (IVIG), or a combination of both, does not seem to improve the ophthalmic outcomes26,27.
Loss of cutaneous pigmentation is common after TEN and may take years to resolve. Other effects include skin scars, onycholysis, and onychodystrophy, which usually resolve within a few months, and loss of nails, thinning of the hair, and pruritus. The reepithelialization process becomes faster with debridement of the areas of the devitalized epidermis and coverage of the stripped areas using biological biosynthetic dressings, silver dressings, or dressings impregnated with antibiotics. Dental complications include oral discomfort, xerostomia, reduced saliva flow, increased salivary acidity, periodontal disease, gingival inflammation, and synechiae. Other complications include respiratory symptoms, such as chronic cough, sinusitis, and laryngeal stenosis in up to 19% of patients20.
In a recent study, necrosis of the entire intraepidermal depth was associated with mortality but was not an independent predictor of death based on the SCORTEN scores. The severity of the dermal infiltrate was not associated with hospital mortality8. A European directive recommends the early use of IVIG after the diagnosis of TEN (recommendation grade C)28, primarily based on a retrospective multicenter study showing that 88% of the patients survived with the use of IVIG with a much higher rate than expected13,26.
The care of skin lesions should be performed at a center with staff qualified to treat burns, as its appropriate management reduces the number of infectious complications.
The availability of a multidisciplinary team with ophthalmologists, uro/gynecologists, intensivists, dermatologists, and plastic surgeons among others is essential for good management and outcome of the case, reducing the rates of sequelae and mortality.
CONCLUSION
TEN is a disease with a severely poor prognosis and must be promptly recognized. For this reason, the physician must know its manifestations and maintain a high level of suspicion. With early diagnosis, risk stratification using the SCORTEN, and appropriate institutional care, mortality can be reduced and sequelae mitigated.
COLLABORATIONS
FCSN Analysis and/or interpretation of data; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
PSP Analysis and/or interpretation of data; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
JMA Analysis and/or interpretation of data; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
LPS Analysis and/or interpretation of data; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
LTC Analysis and/or interpretation of data; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
COU Analysis and/or interpretation of data; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
MPO Analysis and/or interpretation of data; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; and drafting of the manuscript or critical review of its contents.
REFERENCES
1. Becker DS. Toxic epidermal necrolysis. Lancet. 1998;351(9113):1417-20. PMID: 9593426 DOI: http://dx.doi.org/10.1016/S0140-6736(97)11369-1
2. Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68(11):355-61. DOI: http://dx.doi.org/10.1111/j.1365-2133.1956.tb12766.x
3. French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome: our current understanding. Allergol Int. 2006;55(1):9-16. PMID: 17075281 DOI: http://dx.doi.org/10.2332/allergolint.55.9
4. Bulisani ACP, Sanches GD, Guimarães HP, Lopes RD, Vendrame LS, Lopes AC. Síndrome de Stevens-Johnson e necrólise epidérmica tóxica em medicina intensiva. Rev Bras Ter Intensiva. 2006;18(3):292-7. DOI: http://dx.doi.org/10.1590/S0103-507X2006000300012
5. Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56(2):181-200. PMID: 17224365 DOI: http://dx.doi.org/10.1016/j.jaad.2006.04.048
6. Valeyrie-Allanore L, Bastuji-Garin S, Guégan S, Ortonne N, Bagot M, Roujeau JC, et al. Prognostic value of histologic features of toxic epidermal necrolysis. J Am Acad Dermatol. 2013;68(2):e29-35. DOI: http://dx.doi.org/10.1016/j.jaad.2011.10.007
7. Firoz BF, Henning JS, Zarzabal LA, Pollock BH. Toxic epidermal necrolysis: five years of treatment experience from a burn unit. J Am Acad Dermatol. 2012;67(4):630-5. PMID: 22285617 DOI: http://dx.doi.org/10.1016/j.jaad.2011.12.014
8. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69(2):187e1-16.
9. Oliveira FL, Silveira LK, Morais TS, Serra MCVF. Necrólise epidérmica tóxica e síndrome de Stevens Johnson: atualização. Rev Bras Queimaduras. 2012;11(1):26-30.
10. Cartotto R, Mayich M, Nickerson D, Gomez M. SCORTEN accurately predicts mortality among toxic epidermal necrolysis patients treated in a burn center. J Burn Care Res. 2008;29(1):141-6. DOI: http://dx.doi.org/10.1097/BCR.0b013e31815f3865
11. Trent JT, Kirsner RS, Romanelli P, Kerdel FA. Use of SCORTEN to accurately predict mortality in patients with toxic epidermal necrolysis in the United States. Arch Dermatol. 2004;140(7):890-2. PMID: 15262712 DOI: http://dx.doi.org/10.1001/archderm.140.7.890
12. Downey A, Jackson C, Harun N, Cooper A. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. 2012;66(6):995-1003. PMID: 22169256 DOI: http://dx.doi.org/10.1016/j.jaad.2011.09.029
13. Comparin C, Hans Filho G, Takita LC, Costa Nde C, Nascimento RA, Nanni Lde O. Treatment of toxic epidermal necrolysis with intravenous immunoglobulin: a series of three cases. An Bras Dermatol. 2012;87(3):477-81. DOI: http://dx.doi.org/10.1590/S0365-05962012000300022
14. Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128(1):35-44. PMID: 17805350 DOI: http://dx.doi.org/10.1038/sj.jid.5701033
15. Ko TM, Chung WH, Wei CY, Shih HY, Chen JK, Lin CH, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011;128(6):1266-76.e11. DOI: http://dx.doi.org/10.1016/j.jaci.2011.08.013
16. Criado PR, Criado RFJ, Vasconcellos C, Ramos RO, Gonçalves AC. Reações cutâneas graves adversas a drogas - aspectos relevantes ao diagnóstico e ao tratamento - Parte I - Anafilaxia e reações anafilactóides, eritrodermias e o espectro clínico da síndrome de Stevens-Johnson & necrólise epidérmica tóxica (Doença de Lyell). An Bras Dermatol. 2004;79(4):471-88. DOI: http://dx.doi.org/10.1590/S0365-05962004000400009
17. Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282(5388):490-3. PMID: 9774279 DOI: http://dx.doi.org/10.1126/science.282.5388.490
18. Nassif A, Bensussan A, Dorothée G, Mami-Chouaib F, Bachot N, Bagot M, et al. Drug specific cytotoxic T-cells in the skin lesions of a patient with toxic epidermal necrolysis. J Invest Dermatol. 2002;118(4):728-33. PMID: 11918724 DOI: http://dx.doi.org/10.1046/j.1523-1747.2002.01622.x
19. Palmieri TL, Greenhalgh DG, Saffle JR, Spence RJ, Peck MD, Jeng JC, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehabil. 2002;23(2):87-96. DOI: http://dx.doi.org/10.1097/00004630-200203000-00004
20. Quirke KP, Beck A, Gamelli RL, Mosier MJ. A 15-year review of pediatric toxic epidermal necrolysis. J Burn Care Res. 2015;36(1):130-6. DOI: http://dx.doi.org/10.1097/BCR.0000000000000208
21. Oplatek A, Brown K, Sen S, Halerz M, Supple K, Gamelli RL. Long-term follow-up of patients treated for toxic epidermal necrolysis. J Burn Care Res. 2006;27(1):26-33. PMID: 16566534 DOI: http://dx.doi.org/10.1097/01.bcr.0000194268.01514.f8
22. Yeong EK, Lee CH, Hu FC, M Z W. Serum bicarbonate as a marker to predict mortality in toxic epidermal necrolysis. J Intensive Care Med. 2011;26(4):250-4. DOI: http://dx.doi.org/10.1177/0885066610390466
23. Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nature Med. 2008;14(12):1343-50. DOI: http://dx.doi.org/10.1038/nm.1884
24. Brinca A, Andrade P, Xavier MM, Gonçalo M, Figueiredo A. Síndrome de Stevens-Johnson e Necrólise Epidérmica Tóxica - Casuística de 10 Anos. Rev Soc Port Dermatol Venereol. 2011;69(3):403-12.
25. Heng JS, Malik N, Joshi N, Hayes M, Jones I, Fuller LC, et al. Severity of acute ocular involvement is independently associated with time to resolution of ocular disease in toxic epidermal necrolysis patients. Br J Ophthalmol. 2015;99(2):251-4. DOI: http://dx.doi.org/10.1136/bjophthalmol-2014-305506
26. Kim DH, Yoon KC, Seo KY, Lee HS, Yoon SC, Sotozono C, et al. The role of systemic immunomodulatory treatment and prognostic factors on chronic ocular complications in Stevens-Johnson syndrome. Ophthalmology. 2015;122(2):254-64. PMID: 25262319 DOI: http://dx.doi.org/10.1016/j.ophtha.2014.08.013
27. Morales ME, Purdue GF, Verity SM, Arnoldo BD, Blomquist PH. Ophthalmic Manifestations of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis and Relation to SCORTEN. Am J Ophthalmol. 2010;150(4):505-10e1. DOI: http://dx.doi.org/10.1016/j.ajo.2010.04.026
28. Enk A; European Dermatology Forum Guideline Subcommitte. Guidelines on the use of high-dose intravenous immunoglobulin in dermatology. Eur J Dermatol. 2009;19(1):90-8.
1. Hospital São Lucas, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil
2. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil
3. International Society of Aesthetic Plastic Surgery, Porto Alegre, RS, Brazil
Institution: Hospital São Lucas, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Corresponding author:
Francisco Carlos Santos Neto
Av. Ipiranga, 6690, sala 220 - Jardim Botânico
Porto Alegre, RS, Brazil Zip Code 90610-000
E-mail: chicosantosneto@gmail.com
Article received: December 3, 2015.
Article accepted: April 10, 2016.
Conflicts of interest: none.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license











