

Original Article - Year 2016 - Volume 31 -
Versatility of the mid-forehead flap in facial reconstruction
Versatilidade do retalho médio-frontal nas reconstruções faciais
ABSTRACT
INTRODUCTION: Reconstruction of complex facial defects is a challenge to the plastic surgeon. Different missing anatomical units must be accessed in different ways and with individualized goals, always tailoring the options to the patient's needs. The objective is to examine the role of the mid-forehead flap in the reconstruction of different anatomical facial units.
METHODS: Retrospective analysis of patients who were operated on by the author from February 2010 to June 2015. Patients were evaluated according to age, sex, lesion etiology, defect location, number of operations performed per patient, and postoperative complications.
RESULTS: Fifteen patients (mean age, 69 years) underwent facial reconstruction with a mid-forehead flap. Thirteen patients required more than one operation for pedicle refinement and transection. There was one case of partial necrosis of the flap in the columella region, with satisfactory healing by second intention. There was no infection or hematoma. All secondary cartilage grafts showed integration into the recipient bed.
CONCLUSIONS: The mid-forehead flap remains an important tool for the reconstruction of major facial defects. It allows the transfer of frontal tissue in an efficient and reliable way with minimal deformity in the donor area, resulting in an esthetically acceptable reconstruction.
Keywords: Reconstructive Surgical Procedures; Skin neoplasms; Nose/surgery; Surgical Flaps.
RESUMO
INTRODUÇÃO: A reconstrução de defeitos complexos faciais é um desafio ao cirurgião plástico. Diferentes unidades anatômicas ausentes devem ser acessadas de maneiras distintas e com objetivos próprios, sempre adequando as possibilidades ao paciente em questão. O objetivo é mostrar o papel do retalho médio-frontal na reconstrução de diferentes unidades anatômicas faciais.
MÉTODOS: Análise retrospectiva de pacientes operadas pela autora, no período de fevereiro de 2010 a junho de 2015. Os pacientes foram avaliados em relação à idade, sexo, etiologia da lesão, localização do defeito, número de tempos cirúrgicos realizados por paciente e complicações pós-operatórias.
RESULTADOS: Quinze pacientes foram submetidos à reconstrução facial com retalho médiofrontal, com média de idade de 69 anos. Treze pacientes necessitaram mais de um tempo cirúrgico para refinamento e transecção do pedículo. Houve um caso de necrose parcial do retalho na região da columela, com cicatrização satisfatória por segunda intenção. Não houve infecção ou hematoma. Todos os enxertos cartilaginosos secundários se integraram ao leito receptor.
CONCLUSÕES: O retalho médio-frontal permanece como importante ferramenta na reconstrução de grandes defeitos faciais. Permite a transferência de tecido frontal de forma eficiente e confiável com mínima deformidade na área doadora, possibilitando uma reconstrução esteticamente aceitável.
Palavras-chave: Procedimentos cirúrgicos reconstrutivos; Neoplasias cutâneas; Nariz/cirurgia; Retalhos cirúrgicos.
In the Sanskrit text Sushruta Samhita, considered one of the first major detailed treatises of the study of medicine and surgery, dating back to around 600 A.D., one can find the first known description of nasal reconstruction using the mid-forehead flap1. The surgery, practiced in India, was disseminated by Buddhist missionaries practicing Ayurvedic medicine2.
The first description in English of the mid-forehead flap appeared in the Madras Gazette in 1793. A year later, it was published in the Gentleman's Magazine of London. In 1816, the English surgeon J.C. Carpue reported two successful cases of nasal reconstruction employing the forehead flap1. The classic mid-forehead flap (with vertical orientation, in the midline of the frontal region) was popularized in the US by Kazanjian, in 19463.
The Indian forehead flap lifts tissue from the midline, and its vascularization is based on the bilateral supraorbital and supratrochlear vessels. Its base is drawn at the height of the eyebrows. Its length is limited by the hairline implantation. To reach the nasal region, it undergoes a 180º torsion, which can compromise the blood supply3.
Initially, changes in the design of the flap were made with the aim of compensating for such limitations. Since the forehead height cannot be changed, the flap can be effectively lengthened by modifying its angulation (Auvert in 1850 inclined the flap by 45º)1 and lowering its point of rotation. These modifications reduced the torsion at the base of the pedicle and brought the flap closer to the recipient area3.
The analysis of the facial vasculature encouraged surgeons to identify the anatomical basis of the midforehead flap. Millard, among others, cited the axial blood flow, the reliability, and the design of the flap below the orbital rim as advantages of the paramedian flap2.
Mangold et al., cited in Reece et al.2, showed that the supratrochlear artery follows the paramedian line and anastomoses with the medial branch of the dorsal nasal artery. McCarthy et al., cited in Reece et al.2, demonstrated that the frontal region is perfused by an arcade of supraorbital, supratrochlear, infraorbital, and dorsonasal branches, as well as angular branches of the facial and superficial temporal arteries. A rich anastomotic plexus in the medial canthus allows the viability of a unilateral flap, even after the division of supraorbital, supratrochlear, and infratrochlear vessels.
OBJECTIVE
The objective of this study was to demonstrate the versatility of the mid-forehead flap in the reconstruction of different anatomical facial units. It is important to highlight the need for repeated refinement of surgical procedures until the facial contour and definition are satisfactory.
METHODS
The study was conducted by retrospective analysis of patients who were operated on by the author from February 2010 to June 2015. Patients who were undergoing facial reconstruction with the mid-forehead flap, regardless of the location and size of the defect or the use of other associated flaps/reconstructive techniques required in more complex reconstruction, were selected.
The patients were evaluated according to age, sex, etiology of the injury, location of the defect, the number of operations performed per patient, and postoperative complications.
Surgical Technique
In the first operation, which involved the transfer of the forehead tissue to the recipient area and, in almost all cases, tumor resection, patients underwent general anesthesia. In any subsequent stages, the choice of general or local anesthesia + sedation varied with the surgical plan and the patient; in cases requiring more complex reconstructions involving cartilage grafts in elderly patients or with comorbidities, general anesthesia was preferred. In the case of young patients with coverage defects or those only undergoing resection of the pedicle, local anesthesia + sedation was chosen.
Anatomically, the layers of the frontal region consist of skin, subcutaneous tissue, frontal muscle, and a thin areolar layer. The supratrochlear vessels pass deeply over the periosteum and the supraorbital margin in an upward direction in the muscle to assume an almost subdermal position at the hairline3.
Traditionally, during the first stage of flap transfer, the frontal muscle and subcutaneous tissue are excised distally, regulating the flap for positioning in the donor area. However, as proposed by Menick, resection of the frontal muscle and subcutaneous layer removes the myocutaneous component from the blood supply and exposes the subdermal surface, which is more susceptible to fibrosis and contraction, causing late contour deformities.
This increase in the vascular supply of the flap is particularly important in the case of smoking patients and those undergoing major reconstruction. For this reason, it was decided to raise the flaps in the entire plane, including muscle and subcutaneous tissue in all patients in the study4.
In the event of nasal defects in the entire plane, when located at the nasal ala, the primary technique of repairing the lining used involved the construction of a distal extension in the flap, so that it could be folded at the tip. When the defect was located in the lateral wall of the nose, we usually chose the mucosal flaps of the nasal septum.
In the second surgical stage, the flap is fully elevated again and adjusted to a thickness of 3-4 mm. Cartilage grafts, when needed, are positioned for support. The main donor area of grafts to the nasal tip was the pinna, because it is easily accessible, has a similar conformation to the nasal ala, and presents minimum morbidity at the donor site.
In the event of defects of the alar region or tip, with intact support and lining and/or in elderly patients with serious associated diseases, the flap can be adjusted and the pedicle divided at this second stage, avoiding additional surgical time.
The third stage involves the transection of the pedicle and repositioning of the eyebrows. Additional surgical revisions are used for small refinements, such as retouching of scars, definition of the alar fold, and retouching of the alar rim.
RESULTS
Fifteen patients were selected for analysis: 10 male and 5 female. In 14 patients, the etiology was squamous cell carcinoma; in one case, the patient reported a nasal deformity since birth. The ages ranged from 42 to 85 years (average: 69 years). The postoperative follow-up ranged from 3 months to 1 year.
The number of operations performed ranged from 1 to 4. The determinant factors of the number of operations were: the location of the lesion, the stage of the disease, the age of the patient, and his/her desire for new refinements in the treated region.
Except for the patient with a congenital deformity (Figures 1 to 3), all patients underwent excision of the tumor by the head and neck surgery team. Perioperative frozen sections were performed to ensure complete removal of lesions.
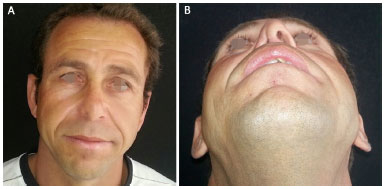
Figure 1. A 42-year-old patient presented with a complaint of nasal obstruction and congenital atresia of the right nostril. A: Front View; B: Basal view.
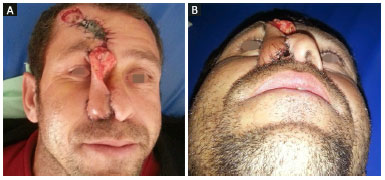
Figure 2. Intermediate phase of paramedian forehead flap transfer. A: Front view; B: Basal view.

Figure 3. Late results with treatment of nasal obstruction; patient showing improvement in the breathing pattern. A: Front view; B: Basal view.
In several patients, the defects resulting from oncologic resection included various anatomical units (Figures 4 to 6), involving different reconstructive techniques, including other local flaps and skin grafting; however, the use of the forehead flap as part of the repair was the common denominator in all patients selected for the study.
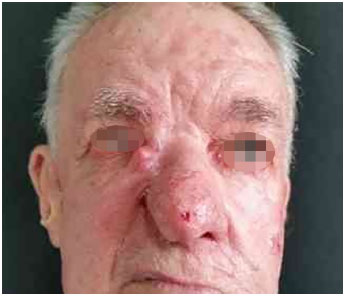
Figure 4. Basal cell carcinoma of the nose: preoperative appearance. Patient with extensive recurrent lesion on lateral nasal wall, nasal tip, and right malar region: preoperative lesion appearance.

Figure 5. A: Basal cell carcinoma of the nose: perioperative appearance. Perioperative view of the defect created after extensive lesion excision, with compromise of the lateral nasal wall, nasal tip in the entire plane, and right malar region, extending to the anterior wall of the maxillary sinus, and communicating with the nasal cavity; B: Bottom view.

Figure 6. Basal cell carcinoma of the nose: postoperative appearance 6 months after nasal reconstruction with paramedian forehead flap folded at the tip for use in lining the alar region. The nasal cavity was separated from the maxillary sinus by the creation of a nasal septum flap. The malar region was closed with a local advanced flap.
All secondary cartilage grafts showed integration into the recipient bed. There were no cases of extrusion or cartilaginous reabsorption. There were no cases of infection or hematoma. There was one case of cutaneous necrosis adjacent to the reconstruction of the columella, with complete healing by second intention after local care and daily dressing. One patient had recurrence of the tumor, which reached the orbital floor (although the frozen section performed during the excision showed clear surgical margins), but refused a new surgical procedure that would involve exenteration of the orbit.
Table 1 summarizes the patients according to age, etiology, anatomic location of the defect, and number of surgeries performed.
DISCUSSION
Many people believe that plastic surgeons are able to restore burned, cut, necrotic, or lost parts of the body to their pre-injury characteristics. A wound can be treated using secondary healing, skin grafts, or different types of flaps, but such restorations do not necessarily re-establish subtleties able to define facial features3.
The prerequisite for facial reconstruction is clear goal planning and commitment to achieve the goal. The complete repair must be thought out, designed step-by-step, and carried out in a sequence of coordinated steps5.
Some general principles must be applied in the repair of complex facial defects6:
1. Molds based on the non-affected contralateral anatomic region allow proper positioning of structures, incisions, and the exact replacement of missing tissues;Although we cannot control scarring or prevent scars, the subunit theory is important, since we can select the color, texture, and thickness of the donor tissue; control the size, shape and position of incisions; and carefully select and modify donor and recipient tissues in a way that will most likely replace the defects, while maintaining contours and characteristics expected for successful reconstruction3.
2. Previous scars usually can be ignored. The main determinant of what is normal is the contour, and this guides the incisions;
3. Alterations of the defect in size, location, shape, and depth may be required to recreate anatomical units;
4. Avoid the "one defect" and "one flap" premise when treating composite defects encompassing multiple facial units;
5. Look for tissues similar to the affected region. Prefer local tissue for coverings and distal tissues for linings/non-apparent areas;
6. Specifically in relation to the nose, the alar subunit should be precisely positioned under a stable platform;
7. Use primary and late primary bone and cartilage grafts for three-dimensional reconstruction of the support;
8. Rebuild in facial units;
9. Use intermediary surgical stages as an advantage;
For hundreds of years, facial defects have been reconstructed with the use of the forehead pedicle flap. Studies by Mangold, McCarthy, and others have described the anatomical basis of the flap, allowing modifications that were responsible for the increase in its scope and reliability2.
Recent studies confirm previous findings that the supratrochlear artery is the main axial supply of the flap, although smaller branches such as the nasal dorsal artery (branch of the angular artery) are capable of ensuring perfusion of the flap2.
The reconstruction of complex nasal defects requires the reconstitution of the nasal mucosa, the osteocartilaginous support, and the skin cover with color, texture, and contour similar to that of the original skin, in addition to restoring normal nasal respiratory function7.
The paramedian forehead flap transfers tissue reliably and efficiently, with minimal deformity in the donor area, and is the most esthetically pleasing option for both the nose and frontal region. The technique allows the surgeon to restore a thin, malleable covering from a thick frontal tissue in two, or preferably three, stages. The maximum possible blood supply is maintained by raising the flap to full thickness in the first operation.
By reflecting the skin of the covering, complete cartilage or bone support can be created through primary or late primary grafts (second operation). At this intermediate stage, all coverage is modified in order to define the nasal subunits. The lining should be thin, malleable, and well vascularized. It can usually be recreated through intranasal mucosal flaps, skin grafts, or the forehead flap bent at the tip. The technique also allows the opportunity to revise imperfections and maximize the contour of the more esthetic distal regions of the nose before the separation of the vascular pedicle3.
The major challenge in the reconstruction of anophthalmic cavities is the three-dimensional design that allows the positioning of the prosthesis in a natural and relatively symmetrical way to the contralateral side8. There are several flaps used to reconstruct the orbital region (Figures 7 to 10); however, in the case of near-total loss of substance, options become more restricted, and the paramedian forehead flap once again is a reliable option9.

Figure 7. Orbital-nasal basal cell carcinoma: preoperative appearance. Preoperative appearance of recurrent nasal basal cell carcinoma in the orbital region, with involvement of the lateral wall of the orbit and extrinsic ocular muscles.

Figure 8. Orbital-nasal basal cell carcinoma: resulting defect.

Figure 9. Orbital-nasal basal cell carcinoma: temporal muscle flap used to fill the orbital cavity.

Figure 10. Orbital-nasal basal cell carcinoma: postoperative appearance one year after excision of nasal basal cell carcinoma in the orbital region, that required exenteration due to involvement of the lateral orbital wall and extrinsic ocular musculature.
Unfortunately, due to oncological reasons, restoration of the anophthalmic cavity to allow prosthetic repair was not an option in the cases presented, with the main concern being only the closure of skin defects.
CONCLUSION
Facial reconstruction surgeries require meticulous preoperative planning. Thus, some questions must be answered, including the surgical objective (simply closure of the defect or restoration close to that of the original), the actual tissue deficiency, whether there is a need to modify the defect size, location, or depth (concept of subunits), how many surgical steps are likely to be required, which donor sites are available, and, in particular, how to modify them for the restoration of each defective anatomical layer to appear as close as possible to the original10.
The paramedian forehead flap allows the transfer of frontal tissue in an efficient and reliable manner, with minimal deformity in the donor area, allowing an esthetically acceptable reconstruction3.
Specifically in relation to nasal reconstruction, despite the availability of other methods, the paramedian forehead flap remains the best option. It allows the surgeon to restore a malleable and nasal tissue-like covering in 2 or preferably 3 operations3.
COLLABORATIONS
MJMC Analysis and/or interpretation of data; statistical analysis; final approval of the manuscript; conception and design of the study; completion of operations and/or experiments; drafting the manuscript or critical review of its contents.
REFERENCES
1. Cintra HPL, Bouchama A, Holanda T, Jaimovich CA, Pitanguy I. Uso do retalho médio-frontal na reconstrução do nariz. Rev Bras Cir Plást. 2013;28(2):212-7.
2. Reece EM, Schaverien M, Rohrich RJ. The paramedian forehead flap: a dynamic anatomical vascular study verifying safety and clinical implications. Plast Reconstr Surg. 2008;121(6):1956-63.
3. Menick FJ. Nasal Reconstruction: Forehead Flap. Plast Reconstr Surg. 2004;113(6):100e-111e.
4. Menick FJ. A 10-year experience in nasal reconstruction with the three-stage forehead flap. Plast Reconstr Surg. 2002;109(6):1839-55.
5. Menick FJ. Defects of the nose, lip, and cheek: rebuilding the composite defect. Plast Reconstr Surg. 2007;120(4):887-98.
6. Menick FJ. Nasal Reconstruction. Plast Reconstr Surg. 2010;125(4):138e-150e.
7. Quintas RCS, Araújo GP, Medeiros Junior JHGM, Quintas LFFM, Kitamura MAP, Cavalcanti ELF, et al. Reconstrução nasal complexa: opções cirúrgicas numa série de casos. Rev Bras Cir Plást. 2013;28(2):218-22.
8. Yanaga H, Mori S. Eyelids and eye socket reconstruction using the expanded forehead flap and scapha composite grafting. Plast Reconstr Surg. 2001;108(1):8-16.
9. Passini AP, Agacy RO, Tissiani LAL, Albano AM. Reconstrução da pálpebra superior com retalho médio-frontal em tempo único associado a tarsorrafia lateral permanente. Rev Bras Cir Plást. 2009;24(2):252-5.
10. Menick FJ. Practical details of nasal reconstruction. Plast Reconstr Surg. 2013;131(4):613e-30e.
Hospital Federal da Lagoa, Rio de Janeiro, RJ, Brazil
Institution: Hospital Federal da Lagoa, Rio de Janeiro, RJ, Brazil.
Corresponding author:
Mayra Joan Marins da Costa
Rua Real Grandeza, 108, sala 110 - Botafogo
Rio de Janeiro, RJ, Brazil Zip Code 22281-034
E-mail: mayrajoan@uol.com.br
Article received: July 28, 2015.
Article accepted: September 29, 2015.
Conflicts of interest: none.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter