

Original Article - Year 2013 - Volume 28 -
A study of physical and organic capsule implants PIP and Rofil taken from 66 patients
Estudo do estado físico e da cápsula orgânica de implantes PIP e Rofil retirados de 66 pacientes
ABSTRACT
BACKGROUND: From the first breast implant surgeries to date, breast implants have advanced in contour, texture, size diversity, and augmentation options. We understand that any deviations from the rigorous techniques designed for breast implants may bring consequences difficult to predict. The purpose of this study is to analyse the physical and organic capsules of PIP and Rofil breast implants removed from 66 patients.
METHOD: We have analyzed 98 pairs of PIP or Rofil breast implants employed in patients from November 2008 to March 2010, of whom 66 were reoperated in the period of February to April 2012.
RESULTS: We have observed an increase in space and presence of material. We have also observed that there were intact breast implants with different aspects and ruptured breast implants with different rupture features and rupture percentage for different brands.
CONCLUSIONS: The main reason for the replacement of breast implants was preventive. The studied brands presented different forms and different percentage of ruptures. Rupture is related to the implantation length of time.
Keywords: Breast implantation. Breast implants. Prostheses and implants. Reoperation.
RESUMO
INTRODUÇÃO: As próteses mamárias, desde o primeiro implante até hoje, evoluíram em conformação, textura, diversidade de tamanhos e opções construtivas. Quando há desvios relacionados à rigorosa técnica com que as próteses mamárias foram concebidas, as consequências são difíceis de prever. O objetivo deste estudo é analisar o estado físico e a cápsula orgânica de implantes mamários PIP e Rofil retirados de 66 pacientes.
MÉTODO: Foram analisadas 98 pacientes portadoras de 98 pares de implantes mamários PIP e Rofil implantados no período de novembro de 2008 a março de 2010, das quais 66 foram reoperadas no período de fevereiro a abril de 2012.
RESULTADOS: Foi observado aumento da loja confeccionada e presença de infiltrado nas pacientes avaliadas. Foram verificados, também, implantes mamários íntegros e implantes mamários rotos com apresentações distintas e porcentagem de ruptura diferente entre as marcas PIP e Rofil.
CONCLUSÕES: O principal motivo para a troca dos implantes mamários foi preventivo. As marcas em estudo apresentaram formas e porcentagens de ruptura diferentes e a ruptura está relacionada com o tempo de implantação.
Palavras-chave: Implante mamário. Implantes de mama. Próteses e implantes. Reoperação.
In 1962, the first silicone breast implant was applied by the American surgeons Thomas Cronin and Frank Gerow1. Since then, breast implant surgery is one of the most common surgical procedures performed annually, in Brazil and worldwide2.
Due to the increase in the rupture rate of the breast implants produced by Poly Implant Prothèse (PIP)3, the French Agency for the Safety of Health Products (Agence Francaise de Securite Sanitaire des Produits de Sante - AFSSAPS) was impelled to inspect this company, noting that the breast implants did not conform to regulations, which led to the suspension of commercialization and use of its products in France. Consequently, the Brazilian National Health Surveillance Agency (ANVISA - Agência Nacional de Vigilância Sanitária) suspended the sales of the breast implants manufactured by the French company on April 1st, 20104. Furthermore, it was announced the Dutch brand Rofil outsourced the silicone gel which filled the PIP implants and was also suspended5.
On February 8, 2012, the Ministry of Health published, in the Oficial Journal of the Union, technical guidelines to guide the replacement for PIP and Rofil silicone implants, which referred that the treatment would be conducted by the Unified Health System (SUS - Sistema Único de Saúde) and by health insurance plans, when proven the need for implant replacement6.
On March 8, 2012, suspecting a deviation in the product composition of the silicone breast implants from the PIP and Rofil brands, the companies Poly Implant Prothèses (PIP) and Rofil Medical Nederland BV, were inspected by ANVISA leading to the suspension of distribution, import, commercialization and implant surgery, with a recall of the remaining products7.
Facing these facts and fearing future problems, the author, who used these implants, during the period between November 2008 and March 2010, in 98 patients, summoned them for a review. This study presents the analysis of the aspects found in the re-operated patients. The removed implants were also analyzed and the results were statistically assessed. The aim of this study was to analyze the physical conditions and the organic capsule of the PIP and Rofil implants removed from 66 patients.
METHODS
During the period between November 2008 and March 2010, the author placed 98 pairs of breast implants of the brands PIP (87 pairs) and Rofil (11 pairs). All the patients were operated on by the author at the Hospital Saúde, located in Guarulhos, SP, Brazil.
The size of these implants varied between 190 ml and 350 ml (Figure 1).

Figure 1 - Implant volume.
The implantation plan for the implants was supramuscular in 92 (93.9%) cases and retromuscular in 6 (6.1%).
After having knowledge of the cancellation of the registration of these implants by ANVISA in January 20128, an attempt was made to contact the 98 patients by telephone and email, and succeeded in 92 cases.
The patients were instructed, informed and invited to attend the clinic for a physical examination and for a breast ultrasound.
Eighty patients attend the clinic for physical examination and none had any complaints, breast alterations or palpable axillary lymph nodes.
The imaging exams were performed in different laboratories by different professionals, according to the release of covenant or the patient's choice. The breast ultrasound reports did not show changes suggestive of rupture, except for one patient.
Based on the French guidelines and the author's opinion, an implant change was proposed.
Of the 98 patients, 66 accepted being submitted to substitution of the implant immediately, 6 were not located, 1 had already substituted the implant by another professional, 6 were pregnant and/or in the postpartum period and 19 did not want to submit to surgery.
Routine electrocardiogram and preoperative laboratory tests were performed. All the patients had surgery between February and April 2012, with local anesthesia and sedation, performed by the same surgery and anesthesia team.
The following routine was used in the surgical procedure for the implant replacement:
For sedation, dosages of midazolam maleate, propofol and fentanyl citrate, were calculated according to patient sensitivity and under the anesthesiologist vigilance.Cleaning of skin with alcoholic chlorhexidine gluconate solution; Skin incision in scar from a previous surgery (lower breast crease); Opening of the implant capsule with electric scalpel and its removal using bidigital maneuver; Cleaning of the implant site with saline solution; Change of surgical gloves by the surgeon at the time of the implant replacement; Handling of the new implant only by the surgeon.
As local anesthesia, lidocaine hydrochloride 100 mg, bupivacaine hydrochloride 25 mg, with adrenaline in milesimal solution 1:200.000 were used.
The inframammary access route was chosen.
The replacement was performed in 66 patients (132 implants), 58 (116 implants) from PIP and 8 (16 implants) from Rofil.
The volumes chosen for the substitution of the new implants were, on average, 65 ml larger, due to their projection equivalence.
In all the breast implant pockets that contained intracapsular fluid, this was collected and measured.Surgical technique performed: Simple replacement (34 patients); Radial capsulotomy (31 patients); Total capsulotomy (1 patient); Replacement from anteromuscular plane to retromuscular plane was performed in 1 patient, due to capsular contracture.
Samples of all operated breast capsules were collected from the inframammary fold region, with a size of 1 cm2, and sent for pathological study to Prof. Dr. Plínio Santos - Anatomia Patológica Ltda.
The average surgery time was 25 minutes, increasing to 60 minutes in the cases where a total capsulotomy was performed, when an apparent macroscopic silicone overflow occurred from the implant and in the patient submitted to the site replacement from anteromuscular to retromuscular.
An antibiotic therapy using ciprofloxacin hydrocloride 500 mg, 12/12 hours, for 7 days, was administered to all patients, starting at induction of anesthesia.
For all patients, a bandage using micropore tape was applied during 15 days, and changed every 4 days.
All patients were discharged from hospital on the same day and received clinical, ultrasonographic and radiographic follow-up for a period of 2 years.
RESULTS
An increase in the primarily handled breast pocket area was observed, furthermore, the peri-implant organic capsule showed an extremely thin thickness.
The ruptured breast implants showed a whitish, dense, odorless and abundant (average of 20 ml) intracapsular infiltrate. In intact breast implant pockets, the majority of the infiltratewas serous, however a whitish infiltrate was also found in 2 sites (Figure 2).
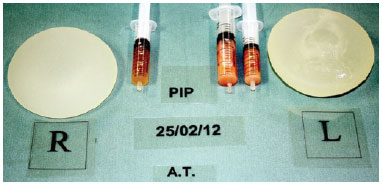
Figure 2 - Implants removed from the same patient. The left implant was ruptured.
Intracapsular serous infiltrate was found in 41 breast implant pockets, intracapsular milky infiltrate in 11 pocketsand, in the remaining 80 pocketsno intracapsular infiltrate was found.
The implants exhibited different aspects, some were yellowish, others looked old or whitish, some looked new (Figure 3) and others had two colorings (Figure 4).

Figure 3 - Aspect of the removed implants.

Figure 4 - Aspect of the removed implants. Two distinct colors are observed in the left implant.
According to the implant brand, two distinct types of ruptures were observed. The PIP breast implants, showed a detachment of the outer blade and the internal silicone gel remained cohesive (Figure 5). The Rofil breast implants that were ruptured showed a leakage of jellified silicone with a non-cohesive appearance (Figure 6).

Figure 5 - Rupture of the PIP implant.

Figure 6 - Rupture of the Rofil implant.
Ruptured implants were found in 7 patients, 4 of Rofil (2 bilateral and 2 unilateral) and 3 of PIP (3 unilateral), representing 37.5% and 2.58% in the incidence of rupture, respectively.
The batches and serial numbers of the implants in question were analyzed and, no correlation was observed between the ruptured breast implants and the batches and/or serial numbers.
The result of the histopathological analysis revealed:
There were no cases of dehiscence, seroma or postoperative infection.Accumulation of amorphous substance (silicone) and xanthomized macrophage clusters in all ruptured breast implant capsules; Presence of histiocytes with large and foamy citoplasm with amorphous substance in one patient with intact implant, suggesting silicone bleeding; Presence of fibrosis in the analysis of the other intact implant capsules.
As postoperative complications there were 4 (6.1%) cases of visible and palpable rippling, who are receiving ambulatory treatment.
DISCUSSION
The French company PIP was responsible for the manufacture of the breast implants registered in Brazil as breast implant filled with high cohesiveness gel registered with ANVISA under the number 80152300001 and imported by the company EMI Import and Distribution Ltd. This French company outsourced its silicone to Rofil Medical Nederland B.V., responsible for manufacturing the Rofil breast implants, registered under the number 8041380002 with ANVISA, and imported by the companies Andema Comercial e Importadora Ltd. and Pharmedic Pharmaceutical Importadora Distribuição e Comércio e Representação5,9.
Imported PIP breast implants totaled 34.631 units, of which 24.534 were marketed until April 2010 and the exact number of Rofil breast implants that entered Brazil, is unknown.
The use of these breast implants in Brazil was suspended, due to the high probability of silicone diffusion through the implant membrane, thus explaining the higher rate of rupture4.
The main causes described in the literature for the breast implant replacement are the occurrence of symptomatic capsular contracture, followed by the patient's dissatisfaction with their breasts appearance11,12. Among the less frequent motives are the occurrences of infection in the implant pocket, implant rupture, presence of breast nodes and/or breast neoplasms11,12.
All the patients included in this study were satisfied with the volume and shape of the breasts, opting for replacement only for fearing the implant was not made with biocompatible material.
The ultrasound was not an efficient method for identifying rupture in the implants.
The incidence of postoperative complications observed in this study (6.1%) is lower than that reported by Handel et al.13 which reached 14.15% of skin ripples with the use of textured implants, 2 hematomas in the immediate postoperative period requiring a reoperation for their drainage, 1 patient with Mondor's syndrome (superficial thrombophlebitis of the breast), with spontaneous resolution after 1 month.
The rupture of the breast implant possibly gave rise to the intracapsular milky fluid in the majority of patients, but the presence of the milky fluid was not exclusive of ruptured breast implants.
There was no correlation between implant volume and rupture rate.
The Dutch breast implant Rofil presented a higher rupture rate than the French breast implant PIP.
In the present study, all the ruptured implants had implantation time higher than 1000 days; however, there were some intact implants with more than 1000 days. In this series, no patient had an implantation time higher than 1250 days.
The postoperative period was less painful than the primary implantation, allowing a faster return to social activities.
CONCLUSION
The physical exams and the ultrasound were not enough to determine the implant replacement. The implantation time is directly related to the implant rupture. Prevention was the main reason for the breast implant replacement. The Rofil implant showed a higher rupture rate when compared to the PIP implant.
REFERENCES
1. Pitanguy I, Barbato C, Dègand M, Lopez CET. Mamoplastia de aumento: considerações sobre a retração capsular. Rev Bras Cir. 1979;69(7/8):237-50.
2. Pitanguy I, Amorim NFG, Ferreira AV, Berger R. Análise das trocas de implantes mamários nos últimos cinco anos na Clínica Ivo Pitanguy. Rev Bras Cir Plást. 2010;25(4):668-74.
3. ANVISA. Alerta 1015 de 01/04/2010.
4. ANVISA. Resolução RE 1558/2010 de 01/04/2010.
5. ANVISA. Informe atualizado sobre a prótese mamária Rofil de 10 de janeiro de 2012.
6. ANVISA. Portaria 196 DOU 08/02/2010.
7. ANVISA. Resolução nº 1016 de 08/03/2012 DOU nº 48 09/03/2012.
8. ANVISA. Informe sobre a reunião entre a Anvisa e agência francesa de 5 de janeiro de 2012. Disponível em: http://s.anvisa.gov.br/wps/s/r/vrK Acesso em 12/3/2012.
9. ANVISA. Informações sobre o implante mamário Poly Implant Prothese (PIP) de 23 de dezembro de 2011. Disponível em: http://s.anvisa.gov.br/wps/s/r/wjh Acesso em 12/3/2012.
10. ANVISA. ANVISA cancela registro de próteses mamárias PIP de 2 de janeiro de 2012. Disponível em: http://s.anvisa.gov.br/wps/s/r/vrP Acesso em 12/3/2012.
11. Cunningham B. The Mentor Core Study on Silicone MemoryGel Breast Implants. Plast Reconstr Surg. 2007;120(7 Suppl 1):19S-32S.
12. Slavin AS, Greene AK. Augmentation mammoplasty and its complications. In: Thorne CH, Chung KC, Gosain AK, Gurtner GC, Mehrara BK, Rubin JP, eds. Grabb & Smith's plastic surgery. 6th ed. Philadephia: Lippincott-Raven; 2007.
13. Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117(3):757-67.
Plastic surgeon, full member of the Brazilian Plastic Surgery Society, São Paulo, SP, Brazil
Correspondence to:
Ana Paula Chiaradia Finamor de Souza
Rua Vergueiro, 2.253 - cj. 218 - Vila Mariana
São Paulo, SP, Brazil - CEP 04101-000
E-mail: finamor@globo.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: 21/6/2012
Article accepted: 6/12/2012
This work was performed at the author's private clinic, São Paulo, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter