

Original Article - Year 2013 - Volume 28 -
Otoplasty using combined techniques: analysis of results and changes in patient quality of life
Correção de orelha de abano por técnica combinada: análise de resultados e alteração da qualidade de vida
ABSTRACT
INTRODUCTION: Prominent ears are relatively frequent and are the most common congenital ear deformity. Numerous otoplasty procedures are described in the literature, including some that recommend the use of combined techniques. Outstanding ears cause considerable aesthetic alteration of the facial symmetry and are associated with psychological and behavioral problems. Thus, it is important to assess the improvement in patient quality of life that this surgical procedure provides. This study aimed to analyze the results of otoplasty procedures using combined techniques and assess the improvement in patient quality of life after the procedure.
METHOD: A retrospective analysis of the results of otoplasty procedures conducted between February 2010 and June 2012 using combined Stenstroem, Mustardé, and Furnas techniques was performed. Epidemiological data and incidence of complications were analyzed, and improvement of quality of life was assessed using the Glasgow benefit inventory questionnaire.
RESULTS: Forty patients were included in the study, corresponding to a sample of 77 ears subjected to surgery. The patients' mean age was 24.4 years. Of the patients, 80% were women. Seven complications (9%) were observed in 7 patients who underwent surgery. The Glasgow benefit inventory questionnaire was answered by 26 patients. The mean score for overall benefit was 62.45 points (range, 30.5-97.2 points).
CONCLUSIONS: The use of the combined techniques in otoplasty showed low incidence of complications and yielded results similar to those described in the literature. Moreover, this study demonstrated that this procedure has the potential to improve the quality of life of patients with prominent ears.
Keywords: Ear, external/surgery. Surgery, plastic/methods. Ear diseases/surgery. Ear cartilage.
RESUMO
INTRODUÇÃO: Orelhas proeminentes são relativamente frequentes, sendo a alteração congênita mais comum da orelha. Inúmeros procedimentos de otoplastia são descritos na literatura, dentre eles alguns que preconizam a utilização de técnicas combinadas. A orelha de abano, além de proporcionar considerável prejuízo estético para a harmonia facial, está relacionada a problemas psicológicos e de comportamento. Assim, torna-se importante a mensuração da melhora da qualidade de vida proporcionada por esse procedimento cirúrgico. Este estudo teve por objetivo analisar os resultados das otoplastias utilizando técnica combinada e avaliar a melhora na qualidade de vida advinda após esse procedimento.
MÉTODO: Análise retrospectiva dos resultados das otoplastias realizadas no período de fevereiro de 2010 a junho de 2012, com técnica combinada de Stenstroem, Mustardé e Furnas. Foram analisados dados epidemiológicos e incidência de complicações, bem como foi avaliada a melhora da qualidade de vida pelo questionário Glasgow Benefit Inventory.
RESULTADOS: Quarenta pacientes foram incluídos no estudo, correspondendo a uma amostra de 77 orelhas operadas. A média de idade dos pacientes foi de 24,4 anos, sendo 80% do sexo feminino. Ocorreram 7 complicações na amostra (9%) em 7 pacientes operados. O questionário Glasgow Benefit Inventory foi respondido por 26 pacientes. A pontuação de benefício geral revelou uma média de 62,45 pontos (30,5 pontos a 97,2 pontos).
CONCLUSÕES: A utilização de técnicas combinadas na correção da orelha de abano revelou baixa incidência de complicações, com resultados comparáveis aos da literatura. Este estudo demonstrou ainda que a realização desse procedimento apresenta a possibilidade de modificar positivamente a qualidade de vida dos pacientes operados.
Palavras-chave: Orelha externa/cirurgia. Cirurgia plástica/métodos. Otopatias/cirurgia. Cartilagem da orelha.
Prominent ears, also known as outstanding ears, are the most common congenital deformity of the auricle, affecting 5% of the Caucasian population1. The condition affects men and women in the same proportion and is genetically inherited in an autosomal dominant manner with variable penetrance2. The development of the auricle occurs rapidly in the first decade of life. In the first year of life the ear reaches approximately 75% of its maximum3; by age 3 years, it reaches 85% of the size of an adult ear and 90% to 95% by age 5 years4. This deformity is diagnosed at birth in approximately 60% of cases; however, it becomes more apparent during the first years of life5,6.
Detailed understanding of the ear's anatomy and development is essential for the surgical planning of the correction of the described anomaly. Therefore, to obtain satisfactory results, embryological and anatomical aspects should be addressed, as well as adequate aesthetic patterns1,7.
The auricle has a unique structure, formed by involutions, furrows, and folds. It is composed of five main elements, namely the concha, helix, antihelix, tragus, and lobule. The anatomical parts of the ear are based on its embryology. The development of the external ear starts on the sixth week of gestation and derives from the first and second branchial arches, with the hyoid arch contributing the most, leading to the formation of the helix, antihelix, concha, antitragus, and lobule. Meanwhile, the mandibular arch contributes to the formation of the tragus and auricular crus1,4.
The arterial supply of the ear comes from the external carotid artery, including the superficial temporal, posterior auricular, and occipital arteries. The veins drain into the posterior auricular, external jugular, superficial temporal, and retromandibular veins. Sensitive innervation is provided by the greater auricular nerve, auriculotemporal nerve (mandibular nerve), lesser occipital nerve, and branches of the cranial nerves VII (posterior and temporal auricular branches), IX, and X. Lymphatic drainage of the ear is divided into the areas of the branchial arches, with the 3 anterior prominences draining to the periparotid lymph nodes and thus to the chain of the anterior triangle of the neck. The 3 posterior prominences drain the retroauricular area to the occipital and mastoid lymphatic chains (and thus to the posterior cervical triangle)8.
The external ear usually has an ovoid shape, with its vertical axis inclined approximately 20º posteriorly (ranging from 15º to 30º). The mean vertical length varies between 5.5 and 6.5 cm, and the width corresponds to 55% of the height (3-4.5 cm). The superior and inferior margins are at the level of the eyebrow and base of the columella, respectively9. Moreover, some parameters are deemed as aesthetically ideal. The auriculocephalic angle, formed by the intersection of a line parallel and tangent to the temporal bone and ear, should be approximately 25º to 30º ideally4,9,10. In prominent ears, this angle typically exceeds 40º to 45º. The scaphoconchal angle, formed by the intersection of a line parallel to the scapha and another parallel to the posterior surface of the concha, is deemed ideal when it is close to 90º. An absent or weak (angle > 90º) scaphoconchal angle, which forms the fold of the antihelix, also contributes to the formation of prominent ears10,11. Another important parameter is the relation between the helix and antihelix. In frontal view, the helix should be completely visible and at a lateral distance of 2 to 5 mm from the antihelix. In addition to these parameters, the relationship between the distance of the most lateral portion of the ear and scalp should be determined. On average, the upper third of the helix is 1 to 1.2 cm from the scalp; the middle third is 1.6 to 1.8 cm from the scalp; and the lobule is 2 to 2.2 cm from the mastoid region12.
The most common etiology of prominent ears is the underdevelopment of the antihelix fold, which occurs in two thirds of all cases and prevents adequate definition between the cavity of the concha and scapha, thus resulting in lateral projection of the superior portion of the helix9,13. In addition, increased depth of the concha (concha hypertrophy), which leads to increased cephalo-auricular angle and significant ear protrusion, is fairly frequent. Patients with outstanding ears usually exhibit a combination of these alterations. Moreover, the external ear can exhibit anomalies in the lobule position2,7,14-16.
In 1845, Dieffenbach was the first to describe the correction of prominent ears17. Since then various treatments and techniques have been developed to correct such deformities, including methods for folding, suturing, repositioning, or even excising ear cartilage. The multiplicity of approaches indicates that a single definitive technique for the correction of the aforementioned deformity does not exist1. In the literature, more than 200 techniques are described for the correction of outstanding ears, with the aim of these otoplasty procedures being the restoration of normal anatomical characteristics3,18.
In general, the different surgical techniques used to correct prominent ears are didactically divided according to whether the ear cartilages are sutured only, or dissected or scarified, in addition to techniques that combine these two principles8. We can therefore combine the proposed techniques to form the antihelix, correct the defects of the concha, or change the lobule position12,19.
In 1968, McDowell20 introduced the parameters that need to be achieved in otoplasty; these parameters are still used today. The aims are the elimination of protrusion in the upper third of the ear, positioning of the helix lateral to the antihelix in frontal view, achieving a smooth and regular contour of the helix, and a not too small or distorted postauricular sulcus. In addition, the measurements should be 10 to 12 mm in the upper third of the ear, 16 to 18 mm in the middle third, and 20 to 22 mm in the lower third. Moreover, the two ears should not differ by more than 3 mm in any position1,20. LaTrenta21 suggested the following three goals that should be aimed for in otoplasty: smooth, rounded, and well-defined antihelix surface; a scapho-conchal angle of 90º; and concha or conchomastoidal angle reduction.
Therefore, surgical treatment of prominent ears usually involves the confection of the antihelix fold, correction of the conchal defect, and lobule repositioning, which are the main causes of the anatomical deformities. Several procedures such as sutures, palisade incisions, abrasions, resections, and cartilage repositioning techniques are used for this purpose. In general, surgeons adopt a combination of techniques, depending on the type of ear deformity1,4,16,22,23.
Correction of conchal defects can be performed by removing the fibromuscular tissue of the postauricular sulcus, and rotating and fixating the concha with concha-mastoid sutures using nonabsorbable thread, as described by Furnas24-26, combined, if necessary, with excisional techniques to reduce major conchal hypertrophy23,27-29.
The antihelix fold can be obtained using permanent nonabsorbable conchoscaphal sutures, as recommended by Mustardé30, and by abrading the anterior surface of the antihelix according to the Gibson principle31, wherein the cartilage tends to warp away from the surface being scored, a technique described by Stenstroem32. Lobule repositioning can be achieved by resecting retroauricular skin in the shape of a fish tail33.
Although auricular deformities do not entail physiological or functional changes, they cause considerable alteration of the aesthetic balance of the face. Furthermore, prominent ears can cause psychological trauma, behavioral disturbances, and withdrawal from social life, especially among children and adolescents1. Previous studies have shown that the psychological problems experienced by these patients are related to decreased quality of life, which results in poor performance at school and at work, and in lack of self-confidence, among other behavioral changes34,35.
Although several studies in the literature have assessed objective data on otoplasty, such as clinical outcomes, incidence of complications, and surgical techniques, few have analyzed the impact of this procedure on patients' quality of life, particularly on their social relations36. Questionnaires have been applied and validated for the measurement of these parameters, such as the Glasgow Benefit Inventory (GBI), specifically developed to subjectively assess the changes in the quality of life of patients who have undergone otorhinolaryngological surgical procedures. This questionnaire has been validated and well studied, and represents a sensitive instrument to measure changes in the quality of life of patients who have undergone otoplasty37-39.
Therefore, the aims of this study were to retrospectively analyze the results of otoplasty procedures using the combined techniques of Stenstroem, Mustardé, and Furnas, combined or not with conchal resection, and to evaluate epidemiological data, incidence of complications, and improvement of quality of life related to otoplasty, using the GBI questionnaire.
METHOD
This retrospective study was performed between February 2010 and June 2012. We evaluated consecutive patients with prominent ears who had undergone unilateral and bilateral otoplasty (performed by the same surgeon) using the combination of the techniques described earlier and who were followed up as outpatients for a minimum of 6 months.
One week before surgery, all the patients provided informed consent regarding the proposed procedure. On the day of the surgical procedure, they were photographed in the anterior, posterior, and oblique views. Surgical marking in the antihelix was performed with a demographic pen after applying digital pressure, and a narrow spindle of skin in the retroauricular area, next to the posterior sulcus, was marked after simulation of the rotation of the auricle (Figure 1).

Figure 1 - Surgical marking of a conservative retroauricular fusiform skin resection after simulation of ear rotation.
The surgical procedure was initiated with local antisepsis using a chlorhexidine aqueous solution. The hair was prepared using cap-shaped sterile fields and micropore for patients with long and very thin hair (Figure 2), as well as an ear plug for ear canal protection. Then, anesthetic blockade of the main nerve branches of the ear and infiltration of the mastoid area, posterior auricular surface, and antihelix were performed using a 10-mL solution of 2% lidocaine with adrenaline and 10 mL of ropivacaine at 10 mg/mL. Approximately 6 mL of solution was used in each ear. Local blockade and general anesthesia were performed concomitantly in pediatric patients and patients who were undergoing combined procedures such as otoplasty with rhinoplasty and otoplasty with breast implantation.

Figure 2 - Preoperative demonstration of a patient ready for surgery.
At the start of the procedure, all the patients received weight-adjusted doses of intravenous prophylactic antibiotic (cefazolin) and anti-inflammatory (dexamethasone) drugs. Moreover, they were administered an oral antibiotic (cephalexin) for 5 days after the procedure, nonsteroidal anti-inflammatory agents for 5 days, and analgesics on demand (dipyrone and paracetamol with codeine).
We used the retroauricular access to perform an incision in the spindle and resect the skin according to the preoperative marking to ensure that the resultant scar was positioned in the retroauricular sulcus. Then, the entire posterior surface of the ear was detached from the perichondrial plane and attached to the lateral margin of the helix (Figure 3). In addition, we dissected the mastoid region, resected the musculoligamentous tissue, exposed the periosteum of the mastoid, and created a space for rotation and fixation of the concha (Figure 4). The posterior auricular muscle was preserved in cases of mild and moderate conchal hypertrophy but resected in cases of severe hypertrophy to create a larger space for conchal rotation. Hemostasis was achieved via direct vessel cauterization using a bipolar electrocautery.

Figure 3 - Perioperative demonstration of wide undermining of the posterior aspect of the ear in pericondral layer up to the margin of the helix.

Figure 4 - Perioperative demonstration of mastoid dissection and resection of soft tissues.
First, the antihelix fold was created. An access was created through the helix tail to the anterior surface of the cartilage (Figure 5). The skin of the anterior surface of the ear was detached, exactly at the level of the future fold of the antihelix, using a blunt retractor. Using a scraper, cartilage scarification was performed at the site of the antihelix, until confection of a soft fold was possible, thus avoiding cartilage fracture. Then, we performed conchoscaphal sutures, as described by Mustardé, with three 4.0 clear braided mononylon sutures placed from bottom to top, ensuring that the knots were not too tight to avoid overcorrection of the antihelix. The sutures were placed by inserting 2 insulin needles, according to the simulation of the new antihelix, and compressing it to the anterior surface of the ear (Figure 6), which was maintained during the placement of the conchoscaphal suture to facilitate the exposure of the surgical field (Figure 7). In cases in which we observed a larger scaphoconchal angle in the upper pole, a fourth suture was placed between the triangular fossa and scapha40, and the thread was repaired for subsequent fixation to the temporal fascia to prevent relapse in the upper pole.

Figure 5 - Perioperative demonstration of definition of ante-helical fold by anterior scarring with rasp.

Figure 6 - Perioperative demonstration of scafoconchal suturing defined by transcutaneous needle marks.

Figure 7 - Perioperative demonstration of needle marks and their maintenance during sutures, helping in the procedure.
Conchal protrusion and excess were corrected using concha-mastoid sutures, as described by Furnas, with two 3.0 clear braided mononylon sutures (Figure 8), with or without partial concha resection. Conchal resection was performed in cases in which decreased opening of the external ear canal was observed during preoperative conchal rotation. Fixation to the temporal fascia was performed in cases in which the thread of the suture that was previously placed between the triangular fossa and scapha was repaired. The retroauricular skin was closed with continuous 5.0 mononylon suture.
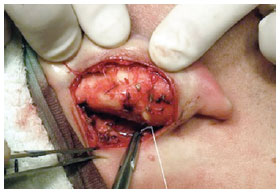
Figure 8 - Perioperative demonstration of conchal protrusion corrected by conchomastoideal sutures, as described by Furnas.
The dressing was performed using damp cotton molded to the depressions and grooves of the ear, gauze, cap-shaped dressing, and bandage, which were removed on the second postoperative day. After removal of the dressing, the patients wore a noncompressive elastic bandage for 15 consecutive days and then only at night for 60 days. The suture of the retroauricular skin was removed on postoperative day 14. The patients were followed up as outpatients, with visits scheduled on postoperative days 7, 14, 30, 90, and 180. They were discharged from follow-up 12 months after the surgical procedure and instructed to come back if necessary.
The epidemiological data analyzed included sex, age, and incidence of complications. In addition, improvement of quality of life after the surgical procedure was assessed via the administration of the GBI questionnaire, which is composed of 18 questions (Figure 9), each scored within a scale from 1 to 5, that can be further divided into three subscales as follows: general, social, and physical health. The GBI questionnaire measures changes in health status caused by surgical interventions. For this measurement, the health status was defined as the perception of general well-being, including psychological, social, and physical health aspects. The questionnaire was retrospectively administered via telephone to patients who had undergone surgery, by a person unknown to them. The obtained score was transported to a scale of benefits, from -100 (maximum harm) to +100 (maximum benefit). A score of zero corresponded to no benefit or no harm.

Figure 9 - Reproduction of the 18 questions of Glasgow Benefit Inventory.
Data analysis was performed using the SPSS version 17.0 software for Windows, considering a P value < 0.05 as statistically significant. The categorical variables were expressed as absolute and percentage values, and the continuous variables were expressed as means or medians, as appropriate, and as minimum and maximum values. The means of changes in quality of life derived from the GBI questionnaire scores in the general scale were analyzed against the zero value, as the latter was considered the preoperative value (no benefit or harm), using the t test for the mean. The study sample was divided into subgroups according to sex, age > 18 years or < 18 years, and occurrence or nonoccurrence of complications for analysis and comparison of quality of life changes according to the GBI questionnaire scores, using the t test for independent samples.
RESULTS
Forty-five patients underwent prominent ear surgical correction using the combined techniques during the study period. Five patients were excluded because the outpatient follow-up was <6 months. Therefore, the sample consisted of 40 patients and 77 ears. The patients' mean age was 24.4 years (range, 8-47 years), and 80% of the patients were women. The median follow-up period was 15.5 months (range, 6-30 months). There were 3 cases of combined otoplasty, namely 2 cases of otoplasty + rhinoplasty and 1 case of breast implant + otoplasty. In most cases (75%), local anesthesia was administered to the patient. Only 2 patients (5%) needed conchal cartilage resection in bilateral otoplasty. The analysis of complications in the 77 operated ears showed a total of 7 complications (9%) as follows: 2 cases of unilateral hematoma (2.6%), which were drained by aspiration puncture during the first revision and had satisfactory progression; 1 case of extrusion of the conchoscaphal suture from the upper pole (1.3%), with 10 months of evolution (the thread was removed without adversely affecting aesthetics); 1 case (1.3%) of unilateral overcorrection (the patient was satisfied and was not interested in a revision); and 3 cases of relapse (3.8%), of which 1 case was a total relapse in one ear after local trauma (ear traction) at 4 postoperative months in a pediatric patient who was reoperated on and had satisfactory progression; and 2 cases of unilateral relapse of the upper pole (one patient was reoperated on 5 months after the initial surgery and diagnosed with opening of the conchoscaphal suture, and the other patient was satisfied with the result and did not want to undergo reoperation). In our study sample, we did not observe any case of infection, hypertrophic scar, necrosis, epidermolysis, or persistent change in sensitivity or pain. Some of the results are shown in the images of Figure 10, which compare the patients' appearances between the preoperative and postoperative phases.
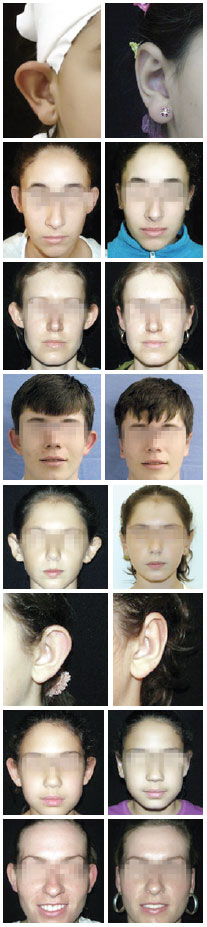
Figure 10 - Pre and postoperative images of cases evaluated in this study.
The GBI questionnaire on quality-of-life changes was administered to 26 of the 40 patients included in the study. The remaining patients were not located or were unable to answer the questionnaire during the period it was administered. The overall mean benefit score was 62.45 points (range, 30.5-97.2 points).
The comparison with the preoperative zero value showed values of P < 0.001 in the overall scale, which demonstrated that the quality of life of these patients improved after the surgical procedure. The comparison between the subgroups showed greater benefit (increased quality of life) for the female patients (65.38 points) than for the male patients (46.5 points), with P < 0.05. The remaining comparisons did not yield statistically significant results.
DISCUSSION
Outstanding ears are determined by one or more anatomical changes. Therefore, adequate surgical planning should separately take into consideration the deformities of each part of the ear to obtain balanced and natural results from the treatment of individual deformities. The ideal result is ears that do not appear to have been operated on16. Thus, conchal hypertrophy, inadequate antihelix formation, or most frequently, the combination of both deformities should be corrected to obtain a satisfactory result. Many authors choose to use a combination of techniques because it allows a complete approach with reproducible and extremely satisfactory results1,16,41.
Studies15 that assessed the incidence of complications and unsatisfactory results have indicated rates ranging from 5.9% to 16.7%, even reaching 60.9%, which is unusual42. In an extensive review of 508 patients operated on in Brazil, the total rate of complications was 24.9%, with some patients having more than one complication43.
Moreover, a meta-analysis published by Limandjaja et al.15 suggests that the incidence of these complications should be measured by the number of ears treated, not by the number of patients operated on. The authors indicate this fact as a cause for the high rate of complications found in some studies, which was the case in our study. It should be noted that complication rates can vary depending on the individual who performs the surgical procedure, with approximately 10% when performed by experienced surgeons and 21.1% (twofold increase) when performed by professionals in training44.
Potential complications can be divided into early and late complications. Among the former, the most common are hematoma, infection, pain, hemorrhage, itching, and necrosis. Late complications are usually diagnosed before the sixth postoperative month and are associated with unilateral or bilateral residual and recurrent deformities, as well as unfavorable healing processes and changes in sensitivity. Complications associated with the suture material, usually in the form of foreign-body granulomas or even extrusion of sutures, have also been reported15,16,45.
In our sample, we did not observe any case of infection, hypertrophic scar, necrosis, epidermolysis, persistent change in sensitivity, or chronic pain; however, cases of hematoma, relapse, hypercorrection, and extrusion of suture thread were diagnosed.
Hematoma is a much feared complication because of the risk of deformity, infection, and chondritis, as well as necrosis of the auricle. Its early identification is mandatory. It is usually diagnosed based on pain that is disproportionate to the operation, with early onset and progression with or without active bleeding through the dressing. Its incidence ranges from 0 and 3.5% of cases15,38,45,46 and may reach 4.2% of cases43. In our study, we found an incidence of 2.6%, which is in line with the literature. Complete resolution was achieved in all the cases after aspiration puncture, and progression was satisfactory, with no cases of chondritis or necrosis observed.
Studies15,16,46-49 indicate partial or complete relapse incidence rates ranging from 0 and 12%. In the present study, the data obtained were consistent with those found in the literature. We observed relapse in 3.8% of the cases, namely partial relapse in 2 operated ears in adult patients (physical examination of the patients clearly showed that the conchoscaphal suture of the upper pole of the ear was loose) and complete relapse in a pediatric patient after local trauma (the ear was pulled by another child, and surgical revision showed the rupture of almost all Mustardé sutures). The relapse rate of the deformity in children is reported to be between 1.8% and 3% and, in most cases, is due to Mustardé suture failure. In adults, relapse occurs most frequently owing to superficial abrasion of the anterior cartilage or posterior Mustardé suture failure4. Some authors state that techniques that exclusively use sutures and spare cartilages entail the loss of correction by 32% to 59%, especially in adults, and recommend overcorrection of the upper pole when these techniques are used2.
In our study, we observed a late case (10 months postoperatively) of suture thread extrusion originating from the Mustardé point in the upper pole of the operated ear; this incidence was similar to that found in the literature. The incidence of this complication can reach 10% of cases15,38,46. The removal of the extruded thread did not negatively affect the final result of the procedure, as had been described by other authors11. We routinely use nonabsorbable thread, as recommended by several authors1; however, we used 3.0 and 4.0 clear braided nylon (Etralon, Biosut, Belo Horizonte, MG, Brazil) because it is soft and prevents the knots being apparent in the ears of patients with very thin skin.
In our study, no cases of infection were observed, which is consistent with results of previous studies46,47. Some studies reported an incidence of postoperative infections of approximately 2.4%, reaching up to 15.5%15. The routine use of preventive antibiotic therapy is controversial; some authors recommend the prophylactic use of antibiotics, whereas others do not, as a great number of studies show similar incidences of infection15, especially when the surgery time is under 2 hours, which is common in this surgical procedure48.
The mean age of our patients was 24.4 years. Nine patients (22%) underwent surgery when they were younger than 18 years. Age varies considerably among studies and is mainly related to geographical and cultural patterns and with the health institution where the procedures are performed. The ideal timing for an otoplasty is controversial; no guidelines have been established that recommend an appropriate age for undergoing the procedure. Most authors describe performing otoplasty in individuals older than 5 or 6 years because at this age, the ear is already the size of an adult ear. Studies have indicated that the consensus among surgeons, parents, and psychologists is that children indicated for otoplasty should be at least 6 years old and preferably able to express their wish to undergo the intervention. It is thus a procedure that is performed to improve the social aspects of the child's life, avoiding name-calling, psychological disturbances, and low self-esteem. Therefore, it is recommended that otoplasty should be scheduled for when the child is older than 5 or 6 years, which coincides with the start of school activity, in order to avoid psychological disturbances2,6,16. However, other studies have demonstrated that otoplasty can be performed before the age of 4 years and that it does not cause problems in the development of the ear50. Moreover, nonsurgical correction can be performed when the deformity is diagnosed immediately after birth, when cartilage is more malleable due to the high levels of circulating maternal estrogens, especially within 3 days after birth, and can be successfully molded using ear molds51.
The analysis of changes in quality of life after the surgical procedure using the questionnaire validated for this purpose37 showed significant improvement in the overall GBI score. These data indicate that this surgical procedure has a positive impact on the self-image of patients with prominent ears, both at the psychological and social levels6. Our overall GBI score result (mean, 62.5) is consistent with that found in the literature; it is even higher than the score obtained in previous studies, which presented means of 30.638 and 37.5 points39. Our study differed from the other studies in that we found a statistically significant difference between the scores of both sexes in the overall GBI scale; the score was higher in the female population (65.3 points) than in the male population (46.5 points). This result was not observed in other studies in which this scale was used to measure the improvement in quality of life of patients who had undergone otoplasty, which may be explained by issues related to the cult of beauty in our society.
CONCLUSIONS
The use of the combined techniques in the correction of prominent ears showed low incidence of complications and reproducible results that were similar to those found in the literature. Accurate preoperative diagnosis of the deformity, and adequate planning and surgical execution are essential for favorable results. Furthermore, this study demonstrated that this procedure can have a positive impact on the quality of life of patients with prominent ears.
REFERENCES
1. Janis JE, Rohrich RJ, Gutowski KA. Otoplasty. Plast Reconstr Surg. 2005;115(4):60e-72e.
2. Adamson PA, Strecker HD. Otoplasty techniques. Facial Plast Surg. 1995;11(4):284-300.
3. Kelley P, Hollier L, Stal S. Otoplasty: evaluation, technique, and review. J Craniofac Surg. 2003;14(5):643-53.
4. Hoehn JG, Ashruf S. Otoplasty: sequencing the operations for improved results. Plast Reconstr Surg. 2005;115(1):5e-16e.
5. Tan ST, Gault DT. When do ears become prominent? Br J Plast Surg. 1994;47(8):573-4.
6. Bradbury ET, Hewison J, Timmons MJ. Psychological and social outcome of prominent ear correction in children. Br J Plast Surg. 1992; 45(2):97-100.
7. Avelar JM. Correção de orelhas em abano. In: Mélega JM, ed. Cirurgia estética. Rio de Janeiro: Guanabara Koogan; 2003. p. 271-80.
8. Petersson RS, Friedman O. Current trends in otoplasty. Curr Opin Otolaryngol Head Neck Surg. 2008;16(4):352-8.
9. Becker DG, Lai SS, Wise JB, Steiger JD. Analysis in otoplasty. Facial Plast Surg Clin North Am. 2006;14(2):63-71.
10. Aygit AC. Molding the ears after anterior scoring and concha repositioning: a combined approach for protruding ear correction. Aesthetic Plast Surg. 2003;27(1):77-81.
11. Adamson PA, Litner JA. Otoplasty technique. Otolaryngol Clin North Am. 2007;40(2):305-18.
12. Cortés W, Glosain AK. Proeminent ears. In: Guyuron B, Eriksson E, Persing JA, eds. Plastic surgery: indications and practice. Philadelphia: Elsevier; 2009. p. 701-16.
13. Owsley TG. Otoplastic surgery for the protruding ear. Atlas Oral Maxillofac Surg Clin North Am. 2004;12(1):131-9.
14. Thorne CH, Beasley RW, Aston SJ, Bartlett SP, Gurtner GC, Spear SL. Grabb & Smith: cirurgia plástica. 6a ed. Rio de Janeiro: Guanabara Koogan; 2009. p. 290-304.
15. Limandjaja GC, Breugem CC, Mink van der Molen AB, Kon M. Complications of otoplasty: a literature review. J Plast Reconstr Aesthet Surg. 2009;62(1):19-27.
16. Yugueros P, Friedland JA. Otoplasty: the experience of 100 consecutive patients. Plast Reconstr Surg. 2001;108(4):1045-51.
17. Dieffenbach JF. Die operative chirurgie, Leipzig F. A. Brockhaus. 1845. Citado por Tanzer RC. Deformities of the auricle. In: Converse JM, ed. Plastic and reconstructive surgery. 2nd ed. Philadelphia: WB Saunders; 1977. p. 1710.
18. Di Cio D, Castagnetti F, Baldasarre S. Otoplasty: anterior abrasion of ear cartilage with dermabrader. Aesthetic Plast Surg. 2003;27(6):466-71.
19. Schlegel-Wagner C, Pabst G, Müler W, Linder T. Otoplasty using a modified anterior scoring technique: standardized measurements of long-term results. Arch Facial Plast Surg. 2010;12(3):143-8.
20. McDowell AJ. Goals in otoplasty for protruding ears. Plast Reconstr Surg. 1968;41(1):17-27.
21. LaTrenta GS. Otoplasty. In: Rees TD, LaTrenta GS, eds. Aesthetic plastic surgery. 2nd ed. Philadelphia: Saunders; 1994. p. 891-921.
22. Adamson PA, Litner JA. Otoplasty technique. Facial Plast Surg Clin North Am. 2006;14(2):79-87.
23. Elliott RA. Otoplasty: a combined approach. Clin Plast Surg. 1990:17(2):373-81.
24. Furnas DW. Correction of prominent ears by conchamastoid sutures. Plast Reconstr Surg. 1968;42(3):189-93.
25. Furnas DW. Otoplasty for prominent ears. Clin Plast Surg. 2002;29(2):273-88.
26. Furnas DW. Otoplasty. In: Aston SJ, Beasley RW, Throne CHM, eds. Grabb and Smith's plastic surgery. 5th ed. Philadelphia: Lippincott-Raven; 1997. p. 431-8.
27. La Trenta GS. Otoplasty. In: Rees TD, LaTrenta GS, eds. Aesthetic plastic surgery. 2nd ed. Philadelphia: WB Saunders; 1994. p. 891-924.
28. Nuara MJ, Mobley SR. Nuances of otoplasty: a comprehensive review of the past 20 years. Facial Plast Surg Clin North Am. 2006;14(2):89-102.
29. Richards SD, Jebreel A, Capper R. Otoplasty: a review of the surgical techniques. Clin Otolaryngol. 2005;30(1):2-8.
30. Mustarde JC. Correction of prominent ears using buried mattress sutures. Clin Plast Surg. 1978;5(3):459-64.
31. Gibson T, Davis W. The distortion of autogenous cartilage grafts: its cause and prevention. Br J Plast Surg. 1958;10:257.
32. Stenstroem SJ. A "natural" technique for correction of congenitally prominent ears. Plast Reconstr Surg. 1963;32:509-18.
33. Rodriguez-Camps S. Our procedure for integral aesthetic otoplasty. Aesthetic Plast Surg. 1997;21(5):332-8.
34. Kapp-Simon KA, Simon DJ, Kristovich S. Self-perception, social skills, adjustment, and inhibition in young adolescents with craniofacial anomalies. Cleft Palate Craniofac J. 1992;29(4):352-6.
35. Bradbury ET, Hewison J, Timmons MJ. Psychological and social outcome of prominent ear correction in children. Br J Plast Surg. 1992;45(2):97-100.
36. Caouette-Laberge L, Guay N, Bortoluzzi P, Belleville C. Otoplasty: anterior scoring technique and results in 500 cases. Plast Reconstr Surg. 2000;105(2):504-15.
37. Robinson K, Gatehouse S, Browning GG. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann Otol Rhinol Laryngol. 1996;105(6):415-22.
38. Braun T, Hainzinger T, Stelter K, Krause E, Berghaus A, Hempel JM. Health-related quality of life, patient benefit, and clinical outcome after otoplasty using suture techniques in 62 children and adults. Plast Reconstr Surg. 2010;126(6):2115-24.
39. Schwentner I, Schmutzhard J, Deibl M, Sprinzl GM. Health-related quality of life outcome of adult patients after otoplasty. J Craniofac Surg. 2006;17(4):629-35.
40. Spira M. Otoplasty: what I do now: a 30-year perspective. Plast Reconstr Surg. 1999;104(3):834-40.
41. de la Fuente A, Sordo G. Minimally invasive otoplasty: technical details and long-term results. Aesthetic Plast Surg. 2012;36(1):77-82.
42. Nielsen F, Kristensen S, Crawford M. Prominent ears: a follow-up study. J Laryngol Otol. 1985;99(3):221-4.
43. Aki F, Sakae E, Cruz DP, Kamakura L, Ferreira MC. Complicações em otoplastia: revisão em 508 casos. Rev Soc Bras Cir Plást. 2006;21(3):140-4.
44. Jeffery SL. Complications following correction of prominent ears: an audit review of 122 cases. Br J Plast Surg. 1999;52(7):588-90.
45. Calder JC, Naasan A. Morbidity of otoplasty: a review of 562 consecutive cases. Br J Plast Surg. 1994;47(3):170-4.
46. Goulart FO, Arruda DSV, Karner BM, Gomes PL, Carreirão S. Correção da orelha de abano pela técnica de incisão cartilaginosa, definição da antélice com pontos de Mustardé e fixação da cartilagem conchal na mastoide. Rev Bras Cir Plást. 2011;26(4):602-7.
47. Ognibene SF, Sperli AE, Freitas JOG, Ognibene SF. Otoplastia com técnica de raspagem de cartilagem auricular e remodelação com pontos no pericôndrio e fixação na mastóide. Rev Bras Cir Plást. 2010;25(2):271-7.
48. Dias GA. Antibioticoterapia profilática e/ou terapêutica em pacientes submetidos à cirurgia plástica estética: uma necessidade? Rev Bras Cir Plást. 2010;25(3):423-7.
49. Bauer BS, Song DH, Aitken ME. Combined otoplasty technique: chondrocutaneous conchal resection as the cornerstone to correction of the prominent ear. Plast Reconstr Surg. 2002;110(4):1033-40.
50. Gosain AK, Kumar A, Huang G. Prominent ears in children younger than 4 years of age: what is the appropriate timing for otoplasty? Plast Reconstr Surg. 2004;114(5):1042-54.
51. Matsuo K, Hirose T, Tomono T, Iwasawa M, Katohda S, Takahashi N, et al. Nonsurgical correction of congenital auricular deformities in the early neonate: a preliminary report. Plast Reconstr Surg. 1984;73(1):38-51.
Physician, Plastic Surgeon, Full Member of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery), Member of the International Society for Plastic Reconstructive and Aesthetic Surgery, Porto Alegre, RS, Brazil
Correspondence to:
Alexander Hornos
Rua Mostardeiro, 5 - cj. 909 - Independência
Porto Alegre, RS, Brazil - CEP 90430-001
E-mail: alexanderhornos@gmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: 27/7/2012
Article accepted: 15/12/2013
Study conducted at the author's private clinic, Porto Alegre, RS, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter