ISSN Online: 2177-1235 | ISSN Print: 1983-5175
Construction of a skin substitute composed of porcine collagen matrix populated with human dermal fibroblasts and keratinocytes: histological evaluation
Construção de substituto da pele composto por matriz de colágeno porcino povoada por fibroblastos dérmicos e queratinócitos humanos: avaliação histológica
Original Article -
Year2012 -
Volume27 -
Issue
4
Cesar Isaac1; Francinni M. P. Rego2; Pedro Ribeiro Soares de Ladeira2; Silvana C. Altram3; Renata C. de Oliveira4; Johnny L. C. B. Aldunate5; André O. Paggiaro6; Marcus Castro Ferreira7
ABSTRACT
BACKGROUND: In the case of extensive lesions, the use of autologous grafts is limited by the extent of the donor area and the clinical condition of patients. Allografts collected from cadavers or volunteers are usually rejected after 1 to 2 weeks, thus serving only as temporary cover for these lesions. Treating major cutaneous lesions with reconstructed autologous skin is an attractive alternative, because it is possible to obtain cultures of cells that multiply rapidly and can be cryopreserved from a small fragment of the patient's skin, thereby facilitating its indefinite use in new treatments. This study evaluated the histological behavior of cultured human keratinocytes and fibroblasts on a collagen matrix derived from porcine small intestinal submucosa.
METHODS: Cells from human epidermis and dermis were grown separately and seeded on porcine collagen matrix, which was maintained in a controlled environment for 21 days before being subjected to histological analysis.
RESULTS: Fibroblasts invaded and colonized the collagen matrix, whereas keratinocytes were organized in laminated and stratified layers on the surface on which they were seeded.
CONCLUSIONS: The use of porcine collagen matrix as a support for human skin cells is feasible, and the organization of these cells resembles the architecture of human skin.
Keywords:
Tissue engineering. Cell culture techniques. Cells, cultured. Burns/therapy.
RESUMO
INTRODUÇÃO: O uso de enxertos autólogos é limitado pela extensão da área doadora e pelo estado clínico dos pacientes, no caso de lesões extensas. Alotransplantes coletados de cadáveres ou voluntários são rejeitados após uma ou duas semanas, servindo apenas como cobertura temporária para essas lesões. O tratamento de grandes lesões cutâneas com tegumento autólogo reconstruído constitui alternativa atraente, já que, a partir de um pequeno fragmento de pele do paciente, pode-se obter culturas de células que se multiplicam rapidamente e podem ser criopreservadas, permitindo, assim, sua utilização em novos tratamentos por tempo indeterminado. Este estudo pretendeu avaliar o comportamento histológico de queratinócitos e fibroblastos humanos cultivados sobre uma matriz de colágeno porcino derivada da submucosa intestinal.
MÉTODO: Células da epiderme e derme humana foram cultivadas separadamente e semeadas sobre matriz de colágeno porcino, onde permaneceram em ambiente controlado por 21 dias, antes de serem submetidas a análise histológica.
RESULTADOS: Observou-se que os fibroblastos invadem e colonizam a matriz de colágeno, enquanto os queratinócitos se organizam de forma laminar e estratificada sobre a superfície em que foram semeados.
CONCLUSÕES: A utilização da matriz de colágeno porcino como carreador de células da pele humana é possível e a organização dessas células se assemelha à arquitetura da pele humana.
Palavras-chave:
Engenharia tecidual. Técnicas de cultura de células. Células cultivadas. Queimaduras/terapia.
INTRODUCTION
Clinical conditions such as burns, trauma, infections, autoimmune diseases, and complex wounds can result in the complete loss of cutaneous tissue1. This dissolution of the skin barrier predisposes patients to infections, increases insensible water loss, and alters thermoregulation, resulting in increased morbidity, prolonged hospitalization, or even death. Allogeneic skin grafting through plastic surgery is one solution for such conditions. However, some patients can have a shortage of skin donor areas. The medical community has still not fully resolved this problem. Therefore, there is currently great interest in biological or synthetic materials that can be used as skin substitutes2.
Skin substitutes are treatment options for patients with major losses of skin and can be human skin or of synthetic origin. Examples include allografts (derived from cadaver skin), xenografts (derived from animal skin), or synthetics constructed by tissue engineering. The decision of which skin substitute to employ is influenced by factors such as the type, size, and depth of the wound; comorbidities; patient preferences; and the surgeon's experience.
The allograft skin can be used to test the therapeutic recipient bed. Good integration increases the chances of autograft success, thus reducing the risk of the loss of scarce autogenous tissues. This can be especially important in patients with major burns or victims of extensive trauma3.
The first attempt to manufacture skin substitutes from epidermal cell cultures was described by Rheinwald & Green4, in 1974; they cultivated a small fragment of healthy skin until a keratinocyte sheet sufficient to cover the wound was produced. Despite the technological innovation associated with the idea of "building skin", this procedure has disadvantages such as a grafting delay of approximately 3 to 4 weeks (the time required to develop the epithelium), fragility, low infection resistance, and high incidence of graft loss. Successful engraftment depends on the presence of residual dermal elements and transport to the wound bed, warranting new research on skin substitutes2.
Skin substitutes are a heterogeneous group of biological and/or synthetic materials that enable temporary or permanent wound closure. Dermal substitutes can be classified as skin xenografts or allografts, or a combination of autologous cultured keratinocytes on dermal matrix produced with the aim of creating a graft more similar to the patient's skin5,6.
Several studies describe the use of both glycerol dermal and decellularized matrices as a scaffold for the development of cultured cells such as keratinocytes and fibroblasts7-9, and even mesenchymal cells10.
Other studies promote different polymers as supporting matrices for these cells11,12. The collagen matrix derived from porcine small intestinal submucosa (OASIS®; Cook Biotech, West Lafayette, USA), which is constituted by a substance similar to human dermis, is widely reported in the literature for its ability to interfere with the healing process13.
This study evaluated the histological behavior of cultured human keratinocytes and fibroblasts on a collagen matrix derived from porcine small intestinal submucosa that was used as a support matrix for these cells.
METHODS
This study was approved by the Ethics Committee for Analysis of Research Projects, CAPPesq, of the Clinic Board of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (Hospital das Clinicas, Faculty of Medicine, University of São Paulo - HCFMUSP). The study was conducted in the Research Laboratory of Cell Culture and Wound Healing (LIM 04) of the Plastic Surgery Department, HCFMUSP (São Paulo, SP, Brazil).
Cell Culture
Normal skin keratinocytes and fibroblasts were obtained from excess skin from 5 female patients aged 20-35 years with Caucasian or African ethnicity.
The skin samples were donated for research purposes during surgical resection and assigned to the Tissue Bank of the Central Institute of HCFMUSP.
Keratinocyte Culture
To isolate keratinocytes, skin fragments were cut and immersed in an enzymatic solution of 0.05% trypsin and 0.02% EDTA (GIBCO, Life Technologies, Baltimore, MD, USA) and maintained with stirring at 37ºC to induce detachment of the cells contained in these fragments. The cell suspension was centrifuged at 1500 rpm for 5 min, and the resultant cell pellet was resuspended in a known volume of Dulbecco's Modified Eagle Medium (DMEM) (GIBCO). The number of cells per mL diluent (cells/mL) was estimated by counting using a Neubauer chamber according to the protocol proposed by Rheinwald & Green14.
All the cells obtained were seeded in 25 cm2 cell culture flasks over a feeder layer composed of irradiated CCL-92 fibroblasts at a ratio of 2 × 106 keratinocytes/25 cm2 and maintained in keratinocyte growth medium (KGM), which contained the following: 60% DMEM (GIBCO), 30% Ham F12 medium (GIBCO), 10% fetal bovine serum (GIBCO), 4 mM glutamine (GIBCO), 0.18 mM adenine (Sigma Chemical - St. Louis, USA), 5 mg/mL insulin (Sigma), 0.4 mg/mL hydrocortisone (Sigma), 0.1 mM cholera toxin (Sigma), 2 mM triiodothyronine (Sigma), 10 ng/mL epidermal growth factor (EGF-Sigma), and penicillin (100 UI/mL) + streptomycin (100 mg/mL) (GIBCO). The culture medium was changed every 48 hours until subconfluence. The cells were then subcultivated by the addition of 0.05% trypsin/0.02% EDTA (GIBCO) for 15 min. The cells of each subcultivation were counted in a hemocytometer.
Fibroblast Culture
Similarly, cells obtained by enzymatic digestion were deposited in 25 cm2 flasks with culture medium for fibroblasts, which contained the following: DMEM supplemented with 10% fetal bovine serum with streptomycin (100 mg/mL), penicillin (100 IU/mL), and amphotericin B (0.25 mg/mL). The vials were stored at 37ºC in an atmosphere of 5% CO2, and the medium was changed every 72 hours until subconfluence, after which the cells were subcultivated.
The assays of cell seeding on the matrix were performed under aseptic conditions in a controlled environment using only sterile materials and reagents.
Secondary Cultures of Keratinocytes and Fibroblasts on Dermal Porcine Collagen Matrices (OASIS®)
Approximately 3 × 105 fibroblasts and 3 × 105 keratinocytes suspended in 1 mL culture medium were plated, individually or together, in stainless steel rings 1 cm in diameter accommodated on 4 cm2 fragments of porcine collagen matrix. After 24 hours, the rings were removed and the culture medium was changed.
Cultures were maintained in an incubator in a controlled environment, and the culture medium was changed every 2 days until day 7 of the study. On day 7, the matrices repopulated by cells were suspended in stainless steel racks in such a manner that only the dermal base stayed in contact with the culture medium and the keratinocytes were exposed to air. This created a condition for cultivation at the air-liquid interface, as proposed by Regnier & Darmon15, in 1989. The culture medium was changed every 2 days until day 14 of the study and daily until day 21, when the protocol was completed.
Fragments of porcine collagen membrane without added cells were maintained under the same conditions to serve as experimental controls.
Histological Analysis
After the specified period, the fragments of collagen matrix with and without cells were preserved in 10% formalin and sent to the Histology Department, Faculdade de Medicina da Universidade de São Paulo (Faculty of Medicine, University of São Paulo), where histological slides stained with hematoxylin-eosin and the picrosirius technique were prepared and analyzed by light microscopy.
RESULTS
The histological appearance of the control matrix (i.e., without added cells) stained with hematoxylin-eosin and picrosirius can be seen in Figure 1.
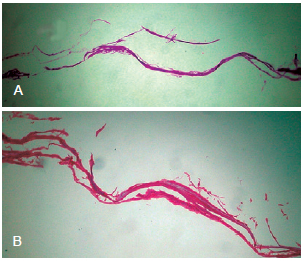
Figure 1 - Light microscopic image of the collagen matrix of porcine intestinal submucosa (OASIS
®) without the addition of cells. In A, hematoxylin-eosin, In B, picrosirius (100×).
Fibroblasts seeded onto the matrix migrated to its interior, populating the collagen network (Figure 2).
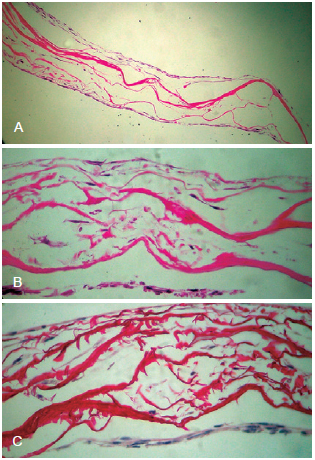
Figure 2 - Light microscopy. In A, dense network of collagen arranged in bundles with interspersed fibroblasts (hematoxylin-eosin, 100×). In B, image indicating the cellular distribution between collagen bundles (hematoxylin-eosin, 400×). In C, image indicating the cellular distribution between the collagen bundles (picrosirius, 400×).
When keratinocytes were seeded on collagen matrix, they organized themselves in a stratified layer (Figure 3).
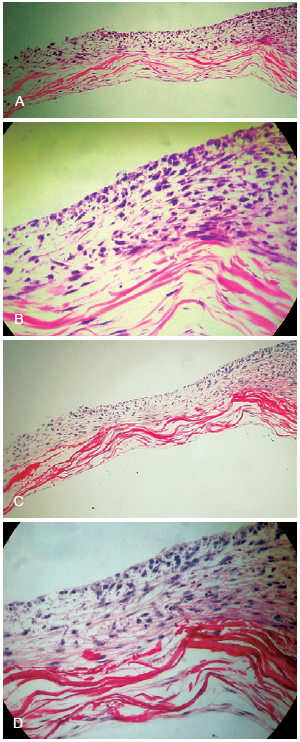
Figure 3 - In A, dense network of collagen arranged in bundles interspersed with fibroblasts and coated by stratified keratinocytes on one side of the matrix after 21 days of culture (light microscopy, hematoxylin-eosin, 100×). In B, image indicating the distribution of fibroblasts between collagen bundles and keratinocyte stratification (light microscopy, hematoxylin-eosin, 400×). In C, dense network of collagen arranged in bundles interspersed with fibroblasts and stratified keratinocytes on one side of the matrix after 21 days (light microscopy, picrosirius, 100×). In D, image indicating the distribution of fibroblasts between collagen bundles and keratinocyte stratification (light microscopy, picrosirius, 400×).
DISCUSSION
Skin substitutes containing keratinocytes and fibroblasts grown on different matrices have been developed6. Models of reconstructed human skin using human or animal dermis, inert matrices, collagen gel, lyophilized collagen, and other polymers filled with melanocytes and/or Langerhans cells are described in the literature. These aim to mimic skin in vivo as well as provide information on cell-cell and cell-matrix interactions, epithelial and dermal cell responses to biological signals and pharmacological agents, and the effects of drugs and growth factors in wound healing10.
Similar to Lazic & Falanga16, we believe that skin substitutes using cultured cells may represent a breakthrough in the clinical and/or surgical treatment of wounds6. Ulcerated lesions persisting for more than 3 weeks exhibit a chronic inflammatory process with the prolonged presence of macrophages, T lymphocytes, and neutrophils. The levels of metalloproteases, which degrade growth factors and adhesion proteins in the extracellular matrix, are also elevated. Furthermore, senescent fibroblasts with defective migration capability and inappropriate responses to growth factors are present in the wound, which originate from a deteriorated extracellular matrix and hinder the healing process17.
There are some commercially available products that aim to modify the characteristics of this extracellular matrix, claiming to improve the wound bed as well as increase the healing and integration of grafts.
One of these products, developed as a result of biotechnological advancements, is a three-dimensional matrix of collagen derived from porcine intestinal submucosa and is approximately 0.15 mm thick (OASIS®, Cook Biotech, Inc. Healthpoint Biotherapeutics, USA); it assists the granulation and epithelialization of lesions, preventing fluid loss and bacterial infection.
Unlike other products, OASIS® has active and intact extracellular matrix components such as glycosaminoglycans (i.e., hyaluronic acid), proteoglycans, fibronectins, basic fibroblast grown factor (FGF), and transforming growth factor beta (βTGF)18.
This study evaluated the behavior of human keratinocytes and fibroblasts seeded on this porcine collagen matrix with the aim of constructing a skin substitute. The ability of the cells to grow on the matrix was assessed using appropriate histological staining, enabling the observation of the resultant phenomena.
Hematoxylin-eosin staining is the main technique for tissue staining in histology. This technique makes it possible to distinguish basophilic (hematoxylin-stained) and acidophilic or eosinophilic (eosin-stained) portions as a result of the attraction between hematoxylin and acidic tissue components such as proteins (rich in amine radicals), nucleus, rough endoplasmic reticulum, and nucleic acids. Moreover, being basophilic, eosin predominantly stains the cytoplasm, collagen fibers, and other structures composed of basic substances19.
Staining with the picrosirius method allows molecules of Sirius Red (acidic) to orient parallel to collagen molecules. Thus, picrosirius staining is specifically used to detect structures composed of oriented collagen molecules20.
The dermo-epidermal compound proposed in this study was not difficult to develop and is easily reproducible; this is because it is a base of simplified dermal tissue with predominantly type I collagen on which fibroblasts and human keratinocytes were seeded. Keratinocyte differentiation occurred during growth of the cultured epithelium, with subsequent stratification of the epidermis. The histological images make it possible to visualize keratin production (Figure 3).
The cultivation of keratinocytes at an air-liquid interface produces a better-developed epithelial structure with more differentiated cells, closer to that found in normal tissue. Despite the formation of confluent and stratified epithelium, some cellular disorganization can be observed, perhaps due to some malformation of the basal membrane. However, electron microscopy studies are required to clarify this.
CONCLUSIONS
This study demonstrates the creation of a dermal-epidermal compound that may become an attractive option for the treatment of burn patients lacking sufficient donor area or patients with chronic wounds, following further tests and modifications.
REFERENCES
1. Ferreira MC, Tuma Jr. P, Carvalho VF, Kamamoto F. Complex wounds. Clinics. 2006;61(6):571-8.
2. Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns. 2007;33(4):405-13.
3. Catena F, Ansaloni L, Gazzotti F, Gagliardi S, Di Saverio S, D'Alessandro L, et al. Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia. 2007;11(1):57-60.
4. Rheinwald JG, Green H. Growth of cultured mammalian cells on secondary glucose sources. Cell. 1974;2(4):287-93.
5. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9):493-508.
6. Paggiaro AO, Isaac C, Bariani G, Mathor M, Herson MR, Ferreira MC. Construção de equivalente dermo-epidérmico. Rev Soc Bras Cir Plást. 2007;22(3):153-7.
7. Herson MR, Mathor MB, Altran S, Capelozzi VL, Ferreira MC. In vitro construction of a potential skin substitute through direct human keratinocyte plating onto decellularized glycerol-preserved allodermis. Artif Organs. 2001;25(11):901-6.
8. Rehder J, Souto LR, Issa CM, Puzzi MB. Model of human epidermis reconstructed in vitro with keratinocytes and melanocytes on dead deepidermized human dermis. Sao Paulo Med J. 2004;122(1):22-5.
9. Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305-13.
10. Pianigiani E, Ierardi F, Mazzanti B, Saccardi R, Cuciti C, Fimiani M. Human de-epidermized dermis as a stem cell carrier. Transplant Proc. 2010;42(6):2244-6.
11. Selim M, Bullock AJ, Blackwood KA, Chapple CR, MacNeil S. Developing biodegradable scaffolds for tissue engineering of the urethra. BJU Int. 2011;107(2):296-302.
12. Shahabeddin L, Berthod F, Damour O, Collombel C. Characterization of skin reconstructed on a chitosan-cross-linked collagen-glycosaminoglycan matrix. Skin Pharmacol. 1990;3(2):107-14.
13. Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D; OASIS Venus Ulcer Study Group. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41(5):837-43.
14. Rheinwald JG, Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975;6(3):317-30.
15. Regnier M, Darmon M. Human epidermis reconstructed in vitro: a model to study keratinocyte differentiation and its modulation by retinoic acid. In Vitro Cell Dev Biol. 1989;25(11):1000-8.
16. Lazic T, Falanga V. Bioengineered skin constructs and their use in wound healing. Plast Reconstr Surg. 2011;127(S1):75S-90S.
17. Moore K, Huddleston E, Stacey MC, Harding KG. Venous leg ulcers: the search for a prognostic indicator. Int Wound J. 2007;4(2):163-72.
18. Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS wound matrix to regranex gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5 Pt 1):258-66.
19. Bancroft JD, Stevens A. Theory and practice of histological techniques. 2nd ed. New York: Churchill Livingstone;1982. p111.
20. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447-55.
1. PhD, Head of the Laboratory of Cell Culture Research and Wound Healing (LIM 04) of the Department of Plastic Surgery, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (Hospital das Clinicas, Faculty of Medicine, University of São Paulo - HCFMUSP), São Paulo, SP, Brazil.
2. Medicine student, Faculdade de Medicina da Universidade de São Paulo (Faculty of Medicine, University of São Paulo), São Paulo, SP, Brazil.
3. Master researcher at the Research Laboratory of Cell Culture and Wound Healing (LIM 04) of the Department of Plastic Surgery, HCFMUSP, São Paulo, SP, Brazil.
4. Biomedical graduate student, Researcher at the Research Laboratory of Cell Culture and Wound Healing (LIM 04) of the Department of Plastic Surgery, HCFMUSP, São Paulo, SP, Brazil.
5. Resident physician of the Department of Plastic Surgery, HCFMUSP, São Paulo, SP, Brazil.
6. PhD in charge of the Tissue Bank of the Central Institute, HCFMUSP, São Paulo, SP, Brazil.
7. Professor of Plastic Surgery, HCFMUSP, São Paulo, SP, Brazil.
Correspondence to:
Cesar Isaac
Avenida Doutor Arnaldo, 455 - sala 1363 - Pinheiros
São Paulo, SP, Brazil - CEP 01246-903
E-mail: cesaris@uol.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Article received: August 17, 2012
Article accepted: October 19, 2012
Work winner of the "Roberto Correa Chem Prize", of the Sociedade Brasileira de Cirurgia Plástica (Brazilian Society of Plastic Surgery), at the 49th Brazilian Congress of Plastic Surgery (2012).
This work was performed in the Laboratory of Cell Culture Research and Wound Healing (LIM 04) Discipline of Surgery, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (Hospital das Clinicas, Faculty of Medicine, University of São Paulo - HCFMUSP), São Paulo, SP, Brazil.
 All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license
All scientific articles published at www.rbcp.org.br are licensed under a Creative Commons license










